6.1: Precursor Chemistry
- Page ID
- 5661
We know that after the Big Bang, there were only a few elements in the Periodic Table: hydrogen, helium, and a sprinkling of lithium. We know that all of the other elements in the Periodic Table were forged during the life and death of stars. We have used spectroscopy to identify the chemical composition of other stars, giant molecular clouds, and even the gas around and between galaxies. The elements in the Periodic Table are ubiquitous.
All of the observations we make of the universe suggest that the laws governing chemistry and physics in our world also operate in the same way on any other planet at any other location in our galaxy and beyond. Therefore, if we can understand the origin of life on Earth, this would gain insights into when, where, and how life might arise on other worlds.
Chemistry to Biochemistry: CHON(PS)
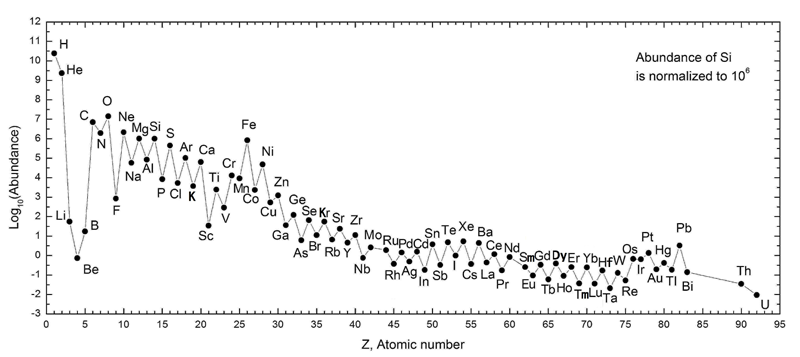
Life is fantastically varied and complex. Yet, all life on Earth is largely composed almost entirely of four elements. Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) together (CHON) make up approximately 98% of all living things. It is probably not a coincidence that these elements are among the most common in the universe (see figure below). To understand what makes CHON so favorable for life, we first consider the chemistry of these elements.
The chemical reactivity of an element is largely dependent on the arrangement of electrons. Electrons are negatively-charged, sub-atomic particles that surround the positively charged nucleus of an atom. Electrons are organized into quantized energy levels, portrayed as shells in the Bohr model of the atom. The truth about electrons and chemical bonding is far more complex and fascinating than implied by the Bohr model, but we adopt this simplification because it is conceptually clean and sufficient for the discussion here. Quantum mechanical rules determine the number of electrons that can reside in each electron shell. Electrons in the outermost, or highest-energy level shell of an atom are valence electrons. Valence electrons affect chemical reactivity of an element because this determines the ease and number of chemical bonds that can form.

The total number of electrons in an electrically neutral atom is equal to the number of protons (the atomic number). As the figure above shows, Hydrogen has only one electron while carbon, nitrogen, and oxygen have 6, 7, and 8 electrons respectively. According to the rules of quantum mechanics, the first electron shell can hold two electrons and the second electron shell can hold up to eight electrons. Being electrically neutral is not the lowest energy state for many atoms. The chemistry of elements is driven by the additional stability that occurs when the valence electron shell is filled.
Hydrogen only needs one electron to complete the first electron shell. If hydrogen simply added an electron, the atom would be negatively charged. Instead, atoms tend to share the electrons that are needed to complete their valence shell. If a shared electron is more tightly held by one atom, then the chemical bond is ionic and resulting molecule will be polar, with a distortion in the electron cloud that makes one atom slightly more negative and the other atom slightly more positive. If sharing of valence electrons is more equitable, the bond is covalent and the molecule is non-polar. Most chemical bonds in biological organisms are covalent bonds. The figure below portrays atoms that are sharing their valence shells to aggregate as molecules. Each atom remains neutral, with a completely filled outer electron shell.

Oxygen, with eight electrons, has two electrons in the first electron shell and six valence electrons in the second shell. Oxygen needs two more additional electrons to fill its outer shell and can form two single or one double bond. Double bonds are shorter and harder to break than single bonds. Because hydrogen and oxygen are so close to a full electron shell, they are chemically aggressive ("highly reactive) in trying to complete their shells. This property makes oxygen very effective in attracting electrons from other atoms.
Nitrogen, with seven electrons, has five valence electrons. This means nitrogen can form up to three bonds: three single bonds, or a single and a double bond, or a triple bond. All thee options are commonly found throughout biochemistry. Oxygen's ability to attract electrons and nitrogen's relatively weak bonding of valence electrons drive many biochemical reactions. Hydrogen, with its one electron, can form bonds wherever shells need to be completed and makes up 59% of elements in life.
Carbon is so central to organic processes that organic chemistry is sometimes defined as the study of compounds that contain carbon. With six total electrons, carbon has four valence electrons occupying its second electron shell, allowing it to form four bonds or a combination of single, double and triple bonds. Stable bonds form elements like carbon monoxide (CO) or carbon dioxide (CO2). Carbon-carbon bonds in particular are easily made and remain very stable, allowing carbon to form the networked lattice in graphite or diamonds, or long, cyclical structures, or long complex hydrocarbon molecules.
Carbon's ability to form four separate bonds at once allows it to acquire the important property of chirality. Chirality is defined as the property of an object that can not be superimposed on its mirror image. The classic example, illustrated below, occurs with your left and right hands. When both hands are palm up, they are mirror images of one another. If you lay your hands flat on a table, it is not possible to slide one hand over the other and match the other hand exactly. For this reason, chirality is often also referred to as "handedness".

Carbon atoms exhibit chirality when they are simultaneously bound to four different things. In such a case, the carbon atom provides a chiral center. Chirality can have a huge impact on the reactivity of a molecule in biological systems. An example is give in the Section below, which discusses chirality in amino acids.
Phosphorus and sulfur, while much less abundant, are nonetheless essential to life as we know it, and warrant an honorable mention with CHON(PS). Phosphorus is involved in forming the borders between cells, the back bone of DNA, and energy storage and transport in cells. Sulfur helps to ensure proteins maintain their shape and carry on important functions.
Alternative Biochemistries
While carbon is clearly favored for Earth-based organisms, other hypothetical biochemistries have been considered by biochemists. One of the most frequently imagined alternatives is silicon-based life. Silicon is similar to carbon in that it also has four valence electrons. However, these electrons are in silicon's third electron shell while carbon's valence electrons appear in its second electron shell. The additional shielding of valence electrons by two inner filled shells of electrons is enough to change the nature of silicon bonds. Because of the valence electrons of silicon are farther from the positively charged nucleus, silicon bonds are weaker than carbon bonds. While carbon can effectively form the complex long molecular chains necessary for life, it is rare to find more than three silicon atoms in a single molecule. Even when they do form, compounds with multiple silicon atom have bonds that are easily disrupted by water. The difference in valence shell energy also makes it harder for silicon atoms to form double or triple bonds. Therefore, silicon molecules seldom exhibit chirality.
Because the valence electrons of silicon are more weakly held to the atom, it is easier for oxygen to bond with silicon, but it is also much harder to break these bonds. Once oxygen has bonded with silicon, it takes a lot of energy to pull it away. Biological processes often require recycling elements into different organic compounds. The difficulty in breaking down silicon-oxygen molecules slows the reaction rates. The structure of silicon-oxygen bonds also renders many important organic molecules unfriendly for life. For instance, we exhale carbon dioxide, CO2, as part of the process that generates energy. The silicon counterpart of CO2, silicon dioxide, is a solid rather than a gas, making it much harder to dispose of than carbon dioxide. In general, silicon tends to form more solid, crystalline structures when compared to its carbon counterparts.
We should not assume that silicon will "never" be a good choice for biochemistry. However, the laws of chemistry, seem to strongly favor carbon-based life, especially in temperature and pressure environments found on Earth. The next time your favorite science fiction movie portrays silicon-based life, remember that it should expel a mouth full of silicon dioxide crystals with every breath.
Amino Acids to Proteins
Amino acids are the building blocks of proteins and proteins regulate the processes that drive life. Amino acids are defined by a specific, relatively simple structure and they are found in every living organism on Earth. All amino acids consist of a central carbon atom forming four bonds to: (1) a hydrogen atom, (2) an amino group (-NH2), (3) a carboxyl group (-COOH), and (4) a changeable side chain (figure below). In organic chemistry, molecules with a carboxyl group are called carboxylic acids. This along with the amino group gives these compounds the name amino acid.

The amino and carboxyl groups on allow amino acids to bond to one another through a process of dehydration with the loss of an -OH group and an -H atom (figures below). During this process, a new peptide bond forms between the carbon and nitrogen atoms. Because these molecules are forged through peptide bonds, strings of amino acids attached together are called polypeptide chains. Polypeptide chains are then folded into proteins.
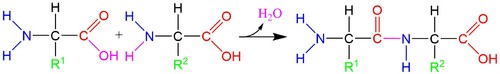
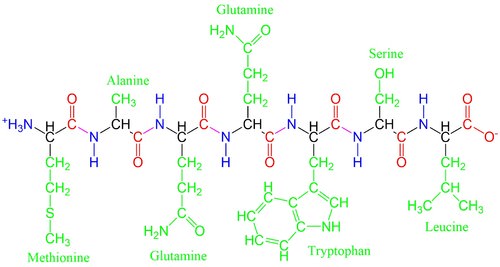
The side chains give each amino acid a unique functionality. Amino acids can be positively or negatively charged, water-repellent, bulky, bent into different configurations, etc, depending on their side chains. These differences help the polypeptides fold as they form proteins, bind to specific compounds, or chemically react in different ways.
Despite the fact that more than 500 amino acids exist on Earth and despite the great variation that is observed in living organisms, life on Earth uses only 20 different amino acids to form the vast array of proteins. Proteins regulate chemical reactions in all aspects of life. This is similar in spirit to the concept that even though the English languages only uses 26 letters, those same letters have been used to write millions of books.
Essential Amino Acids, Body Building, and You
Of the 20 amino acids used by life on Earth, the human body is capable of synthesizing all but nine. These nine amino acids are known as the essential amino acids. It is important to include sources of these amino acids either from meat or plants in a healthy diet since the body has no other source for them.
In fact, many products sell amino acids as supplements targeted towards endurance athletes and body builders. Scientists have been able to trace different amino acids and the role they play in muscle contraction or recovery to identify what the body needs after the coach has said "last set'" for the third set in a row. For example, glutamine is drained during intense physical activity. If the body's glutamine stores become depleted, the body begins to break down muscle cells to compensate.
Prolific Proteins
It is commonly said that you are what you eat, but perhaps more correctly, you are what your proteins decide to do. Proteins are the driving force behind the processes of life. Many proteins act as enzymes, which are highly specified molecules that allow complicated organic reactions to progress more easily. In most organic reactions, the molecules involved must first assume an unfavorable, intermediate configuration (figure below) before progressing to the finished product. Enzymes bind to these starting molecules and act to stabilize the intermediary state. This makes it easier for molecules to progress to the desired, final products. Enzymes are the primary catalyst for organic reactions and they increase reaction rates for biochemical processes.

In addition to acting as enzymes or catalysts, proteins also fulfill several other important roles. Proteins are involved with cell signaling that help different cells in the body work together. Antibodies are proteins that work with the body's immune system to recognize and destroy foreign substances that might cause illness. Structural proteins give shape or rigidity to cells, such as those that make up our nails or hair. Motor proteins allow for the movement of single-celled organisms. In short, proteins are critical for all of the basic functions of life.
Chirality
The central carbon in an amino acid can serve as a chiral center because it is typically bound to four different groups. The one exception is the amino acid, glycine, whose hydrogen side chain makes it a symmetric molecule. This chirality means there exists both left-handed and right-handed amino acids (figure below). Oddly, while either configuration is possible, life on Earth only uses left-handed amino acids.

Studies have been done to investigate the functionality of protein chirality. Synthesis of both left-handed and right-handed amino acids is not only possible, it is chemically equivalent. From an energy standpoint, protein that is composed entirely of right-handed amino acids should function just as well as proteins made of left-handed amino acids. Chirality seems to add an extra layer of regulation in biochemical reactions that may reduce synthesis errors from occurring.
How did life come to pick left-handed proteins over right-handed ones? There are many competing theories as to the origins of this inequality. One idea is that left-handed amino acids are slightly more water soluble and this could have made them easier to incorporate into early life. Alternatively, light in the protoplanetary disk would have been circularly polarized and it is possible that this might have been more damaging to right-handed amino acids or more favorable to left-handed ones. Amino acids found in space, for example on meteorites, also exhibit an excess of left-handed molecules. If polarized sunlight gave rise to this imbalance it could have tipped the scales to the left for life on Earth. Regardless of what established the original inequality, biological processes probably accentuated the imbalance.