6.2: Prebiotic Chemistry
- Page ID
- 5662
Once biochemists understood some of the important chemistry in living organisms, a new field of synthetic biology was born. Biochemists tried to replicate the pathway for the evolution - or the complexification - of simple chemistry.
In 1953, Stanley Miller and Harold Urey published the results of their now famous experiment: they spontaneously produced amino acids from simple elements under conditions emulating early Earth. Given the importance of amino acids and proteins for life, this experiment was viewed as an important step toward understanding the origin of life.
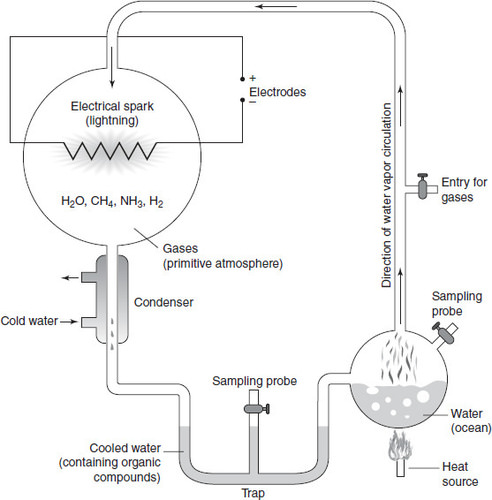
The experimental setup, depicted in the figure above, consisted of two large flasks. One flask contained gases thought to be present in the primitive atmosphere (methane, ammonia, and hydrogen) and the other contained water, representing the ocean. Electrodes were inserted into the atmosphere flask to produce electrical sparks that would emulate lightning.
The ocean flask was heated, producing water vapor that traveled throughout the setup and interacted with gases in the atmosphere and simulated lightning. The enriched vapor then traveled through a condenser and cooled to a liquid state in a u-bend at the bottom of the setup where samples could be collected. Within a day of running the experiment, the liquid at the bottom of this u-bend had turned pink. The inaugural run of the experiment lasted for a week. From these results, Miller was able to identify five different amino acids that had been created from this simple set up of atmospheric gases, lightning, and water alone. As expected, the chirality of these amino acids included both left- and right-handed molecules, even though only left-handed amino acids are incorporated into biology as we know it today. Recent reanalysis of sealed vials have detected more than 20 different amino acids that were actually formed during that one-week experiment.
Since 1953, there have been many additional experiments that aim to synthesize the building blocks of life. Longer chains of amino acids and polypeptides have been synthesized. An impressive experiment was carried out by John Sutherland's lab in 2009. Sutherland began with a compound called 2-amino-oxazole and using phosphorus as a catalyst was able to synthesize some of the nucleotides used in RNA and DNA.
The Murchison Meteorite - amino acids in space
On September 28, 1969, two months after Apollo 11 landed on the moon, a huge meteorite soared through the sky and landed near Murchison, Victoria in Australia. The Murchison Meteorite is one of the most valuable and well-studied meteorites. Importantly, the incoming meteorite was highly visible. It flew through the atmosphere as a fireball and caused a noticeable tremor when it landed. This allowed for rapid recovery of the meteorite, preventing terrestrial contamination. A piece of the Murchison meteorite is shown below.

In total, more than 100 kg (the average weight of an NFL linebacker) of the Murchison Meteorite was recovered over an area of 13 square kilometers. This meteorite contained more than 70 amino acids, many of which are not known to exist on Earth. There is a slight over-abundance of left-handed amino acids, which suggests that there may be a cosmic origin to the dominance of left-handed amino acids that are used by life on Earth. Every amino acid discovered on the Murchison Meteorite has since been successively synthesized using a Miller-Urey-like setup with gases, water, and simulated lightning, demonstrating that these amino acids can spontaneously form under fairly simple conditions. The Murchison Meteorite is proof that amino acids can easily in space. Amino acids: so easy to synthesize that a rock can do it!
Meteors and Meteoroids and Meteorites
The root word, meteor, comes from the Greek word for ''high in the air".
Meteoroids are small rocky bodies in space. They are smaller in size than asteroids or icy comets. When a meteoroid enters the Earth's atmosphere, interactions with the gases in the atmosphere heat up the outer layer, which crumbles away and creates a glowing trail. This light show is what is called a meteor. More colloquially, they are identified as falling stars and are the stuff of Hollywood wishes.
On an average day, 4-8 meteors can be seen every hour, but the number varies greatly by season and time of night. A meteor shower is a time of increased meteors, generally caused when the orbit of the Earth crosses a trail of debris left from passing comets. Since the Earth crosses this material regularly in it's orbit, particular meteor showers occur at the same time every year. The Perseid meteor shower occurs in mid-August and the Leonid meteor shower can be seen in mid-November.
Meteorites are meteoroids that have survived atmospheric heating from the meteor phase and landed on Earth. Most meteors burn up in the atmosphere, so meteorites are relatively rare. Meteor-Wrongs, has a sample of rocks that the were initially believed to be meteorites, but with a terrestrial origin, according to the Department of Earth and Planetary Science at Washington University.
Even more complex organic molecules have been detected in interstellar space, a testament to how easily complex, carbon-based structures can form and remain stable. Some of these molecules have carbon ring-structures, called polycyclic aromatic hydrocarbons and are similar to the organic molecules found in biological organisms. This confirms that carbon is a good base for forming complex molecules structures, including amino acids. While this gives a hint that carbon-based life may also exist on other planets, there are still many steps we don't yet understand about how to go from prebiotic chemistry to life.
Nucleic Acids
RNA (ribonucleic acid) and DNA (deoxyribonucleic acid) are long-chain complex nucleic acids that enable the transfer of information through a genetic code during cell replication. Gene replication and specifically the errors that occur during gene replication allow organisms to be altered so that specific traits that are better adapted to the environment can be selected. Thanks to genetic mutations and natural selection, giraffes have evolved to reach the leaves of tall trees on our planet.
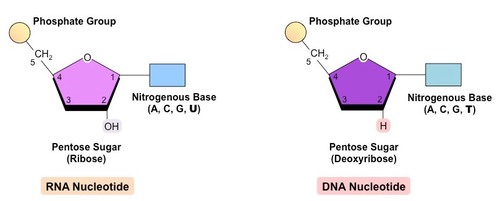
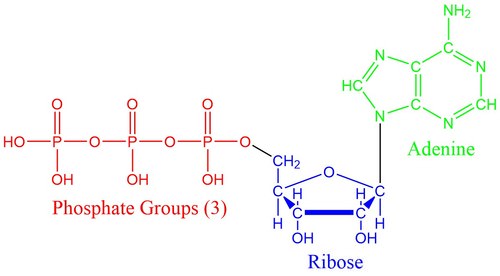
Nucleotides are the building blocks of nucleic acids. As shown above, nucleotides consist of three components: (1) a sugar, (2) a phosphate group, and (3) a base. RNA uses ribose sugar while DNA uses deoxyribose sugar. The phosphate group, adenosine triphosphate (ATP), stores energy for chemical reactions and regulates the enzymatic role of proteins, marking them as active or inactive. There are five different bases that are used. RNA uses adenine, uracil, cytosine or guanine (AUCG) and DNA uses adenine, thymine, cytosine and guanine (ATCG).
Nucleotide bases fall into two categories: double-ringed purines (A,G) and single-ring pyrimidines (C, T, U). These different bases are shown in the figure below. Biologists named some of these bases after the material where they were discovered. For instance, guanine was first discovered from guano, a fancy name for bird poop.

The phosphate group consists of a phosphorus atom bound to four oxygen atoms and contains many high energy bonds. When energy is needed for a chemical reaction, one of the phosphate bonds is broken, converting ATP to ADP, adenosine diphosphate, with only two phosphate groups remaining. ATP is the most widely used energy carrier in all living organisms on Earth.