4.1: Minerals
- Page ID
- 5361
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Are you a mineral?
There used to be a TV commercial that said "you are what you eat." If that's true, then you are partly the mineral halite. That's just table salt, after all.
What is a Mineral?
A mineral is a solid material that forms by a natural process. A mineral can be made of an element or a compound. It has a specific chemical composition. Its chemical composition is different from other minerals. Each type of mineral has physical properties that differ from others. These properties include crystal structure, hardness, density, and color. For example, silver is a soft, shiny metal. Salt is a white, cube-shaped crystal. Diamond is an extremely hard, translucent crystal.
Forms by Natural Processes
Minerals form by natural processes. Minerals are created by processes that happen in or on the Earth. For example, when hot lava cools, mineral crystals form. Minerals also precipitate from water. Some minerals grow when rocks are exposed to high pressures and temperatures.
Could a mineral be made by a process that was not natural? People make gemstones in a laboratory. Synthetic diamond is a common one. But that stone is not a mineral. That's because a mineral must be created by natural processes. This is part of the definition of a mineral.
Inorganic Substance
A mineral is an inorganic substance. It was not made by living organisms. Organic substances contain carbon. Some types of organic substances are proteins, carbohydrates, and oils. Everything else is inorganic. In a few cases, living organisms make inorganic materials. The calcium carbonate shells made by marine animals are inorganic.
Definite Composition
All minerals have a definite chemical makeup. A few minerals are made of only one kind of element. Silver is a mineral made of only silver atoms. Diamond and graphite are both made of only the element carbon.
Minerals that are not just a single element are made of chemical compounds. For example, the mineral quartz is made of the compound silicon dioxide, or SiO2. This compound has one atom of the element silicon for every two atoms of the element oxygen.
Each mineral has its own unique chemical formula. For example, the mineral hematite has two iron atoms for every three oxygen atoms. The mineral magnetite has three iron atoms for every four oxygen atoms. Many minerals have very complex chemical formulas that include several elements. However, even in more complicated compounds, the elements occur in definite ratios.
Solid Crystals
Minerals must be solid. For example, ice and water have the same chemical composition. Ice is a solid, so it is a mineral. Water is a liquid, so it is not a mineral.
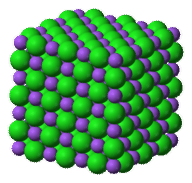
Some solids are not crystals. Glass, or the rock obsidian, are solid. However, they are not crystals. In a crystal, the atoms are arranged in a pattern. This pattern is regular and it repeats. The image below shows how the atoms are arranged in halite (table salt) (Figure below). Halite contains atoms of sodium and chlorine in a pattern. Notice that the pattern goes in all three dimensions.
Sodium ions (purple balls) bond with chloride ions (green balls) to form halite crystals.
The pattern of atoms in all halite is the same. Think about all of the grains of salt that are in a salt shaker. The atoms are arranged in the same way in every piece of salt.
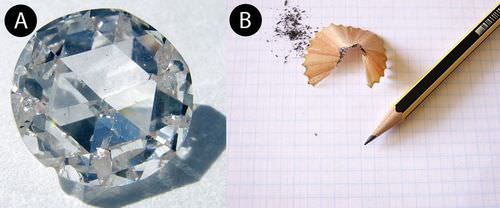
Sometimes two different minerals have the same chemical composition. But they are different minerals because they have different crystal structures. Diamonds are very valuable as gemstones, because they are very pretty and very hard. Graphite is the “lead” in pencils. It's not hard at all! Amazingly, both are made of just carbon. Compare the diamond with the pencil lead (Figure below). Why are they so different? The carbon atoms in graphite bond to form layers. The bonds between each layer are weak. The carbon sheets can just slip past each other. The carbon atoms in diamonds bond together in all three directions. This strong network makes diamonds very hard.
Diamonds (A) and graphite (B) are both made of only carbon, but they're not much alike.
Physical Properties
The patterns of atoms affect a mineral's physical properties. A mineral's crystal shape is determined by the way the atoms are arranged. For example, you can see how atoms are arranged in halite (Figure below). Below, you can see how salt crystals look under a microscope (Figure below). Salt crystals are all cubes whether they're small or large.
Under a microscope, salt crystals are cubes. In this crystal, the corners have worn away.
Physical properties help scientists identify different minerals; this is explored in the concept "Mineral Identification."
Summary
- A mineral is an inorganic, crystalline solid.
- A mineral forms through natural processes. It has a definite chemical composition.
- Minerals can be identified by their characteristic physical properties. They include crystalline structure, hardness, density, breakage, and color.
Review
- What properties does a substance have to have to be a mineral?
- How are diamond and graphite similar? How are they different?
- How is a mineral's shape determined?
Explore More
Use this resource to answer the questions that follow.
- What are the first two criteria for something to be a mineral?
- What is the third thing? What does that mean?
- What is the common name for halite? What is it made of chemically?
- What is true about the elements that make up a mineral?
- Why does halite break into little cubes?
- What is the evidence for an ice cube being a mineral?
- Why isn't glass a mineral?