4.2: Mineral Groups
- Page ID
- 5362
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What would you do with this mineral?
Imagine you are in charge of organizing more than 100 minerals for a museum exhibit. You know that people learn more if they see the minerals together in groups. So how would you group the minerals together in the exhibit? What are some of the possibilities? Size? Shape? Color?
Learn about the minerals behind modern life with this video.
Mineral Groups
Mineralogists are scientists who study minerals. They divide minerals into groups based on chemical composition. Even though there are over 4,000 minerals, most minerals fit into one of eight mineral groups.
Silicate Minerals
Silicates are the largest mineral group. About 1,000 silicate minerals are known. Silicate minerals are also extremely common. They make up over 90% of Earth's crust!
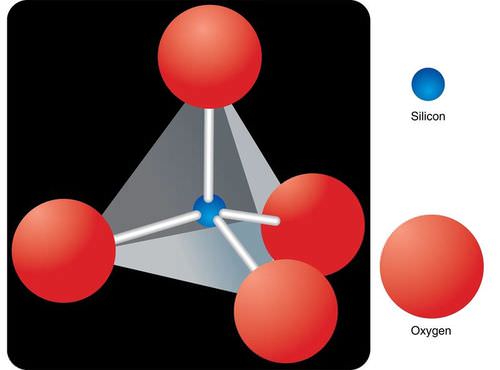
Silicates contain silicon atoms and oxygen atoms. One silicon atom is bonded to four oxygen atoms. These atoms form a tetrahedron (Figure below). The silica tetrahedron is the building block of silicate minerals. Most silicates contain other elements. These elements include calcium, iron, and magnesium.
One silicon atom bonds to four oxygen atoms to form a pyramid
Silicate minerals are divided into six smaller groups. In each group, the silicate pyramids join together differently. The pyramids can stand alone. They can form into connected circles called rings. Some pyramids link into single and double chains. Others form large, flat sheets. Some join in three dimensions.
Feldspar and quartz are the two most common silicates. In beryl, the silicate pyramids join together as rings. Biotite is mica. The silicate pyramids come together to create thin, flexible sheets. Compare the beryl and the biotite pictured below (Figure below).
Beryl (a) and biotite (b) are both silicate minerals.
Native Elements
Native elements contain only atoms of one type of element. They are not combined with other elements. There are very few examples of these types of minerals. Some native elements are rare and valuable. Gold, silver, sulfur, and diamond are examples.
Carbonates
What do you guess carbonate minerals contain? If you guessed carbon, you would be right! All carbonates contain one carbon atom bonded to three oxygen atoms. Carbonates may include other elements. A few of these are calcium, iron, and copper.
Carbonate minerals are often found where seas once covered the land. Some carbonate minerals are very common. Calcite contains calcium, carbon, and oxygen. Have you ever been in a limestone cave or seen a marble tile? Calcite is in both limestone and marble. Azurite and malachite are also carbonate minerals. They contain copper instead of calcium. They are not as common as calcite. Malachite and azurite are used in jewelry; as you can see, they are very colorful (Figure below).
(a) The deep blue mineral is azurite and the green is malachite. (b) This red rhodochrosite is a carbonate mineral. Rhodochrosite is usually pink; the red crystals are rare.
Halides
Halide minerals are salts. They form when salt water evaporates. This mineral class includes more than just table salt. Halide minerals may contain the elements fluorine, chlorine, bromine, or iodine. Some halide minerals combine with metal elements. Common table salt is a halide mineral that contains the elements chlorine and sodium. Fluorite is a type of halide that contains fluorine and calcium. Fluorite can be found in many colors. If you shine an ultraviolet light on fluorite, it will glow!
Oxides
Earth’s crust contains a lot of oxygen. The oxygen can combine with other elements to create oxide minerals. Oxides contain one or two metal elements combined with oxygen. Oxides are different from silicates, because they do not contain silicon. Many important metals are found as oxides. For example, hematite and magnetite are both oxides that contain iron. Hematite (Fe2O3) has a ratio of two iron atoms to three oxygen atoms. Magnetite (Fe3O4) has a ratio of three iron atoms to four oxygen atoms. Notice that the word “magnetite” contains the word “magnet." Magnetite (Figure below) is a magnetic mineral.
Magnetite is one of the most distinctive oxides since it is magnetic.
Phosphates
Phosphate minerals have a structure similar to silicates. In silicates, an atom of silicon is bonded to oxygen. In phosphates, an atom of phosphorus, arsenic, or vanadium is bonded to oxygen. There are many types of phosphate minerals, but phosphate minerals are rare. The composition of phosphates is complex. For example, turquoise contains copper, aluminum, and phosphorus (Figure below).
Turquoise is a phosphate mineral with a beautiful blue color. The stone is not as rare as some minerals and is commonly used for jewelry.
Sulfates
Sulfate minerals contain sulfur atoms bonded to oxygen atoms. Like halides, they can form in places where salt water evaporates. Many minerals belong in the sulfate group, but there are only a few common sulfate minerals. Gypsum is a common sulfate mineral that contains calcium, sulfate, and water. Gypsum is found in various forms. For example, it can be pink and look like it has flower petals. However, it can also grow into very large white crystals. Gypsum crystals that are 11-meters-long have been found. That is about as long as a school bus! Gypsum forms at the Mammoth Hot Springs in Yellowstone National Park (Figure below).
Gypsum is the white mineral that is common around hot springs. This is Mammoth Hot Springs in Yellowstone National Park.
Sulfides
Sulfides contain metal elements combined with sulfur. Sulfides are different from sulfates. They do not contain oxygen. Pyrite is a common sulfide mineral (Figure below). It contains iron combined with sulfur. Pyrite is also known as “fool’s gold.” Gold miners have mistaken pyrite for gold, because pyrite has a greenish gold color.
Pyrite has a similar color to gold. The appearance of the striations make pyrite different from gold.
Summary
- Silicates, made of building blocks of silica tetrahedrons, are the most abundant minerals on Earth.
- Silica tetrahedrons combine together in six different ways to create rings, single and double chains, large flat sheets, or 3-dimensional structures.
- Other mineral groups have other chemical components like carbonates, oxides, or phosphates.
- Minerals that are native elements are made of only one element.
Review
- On what basis are minerals divided into groups?
- What are the chemical characteristics of the most common mineral group?
- How are the silicates categorized?
Explore More
Use the resource below to answer the questions that follow.
- How are minerals classified?
- What do silicate minerals contain?
- Where are silicate minerals found?
- List three examples of silicates.
- What do non-silcate minerals contain?
- What is a native element?
- What are native elements used in?
- List examples of carbonates.
- What are halide minerals used for?
- What are oxides used for?
- What are sulfate minerals used for?
- What are sulfide minerals used for?