10.25: Causes of Air Pollution
- Page ID
- 5502
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Why don't we see emissions like this too often any more?
This photo of a power plant was taken before emission control equipment was added. Emissions are down since laws have been enacted to protect the air.
Causes of Air Pollution
Most air pollutants come from burning fossil fuels or plant material. Some are the result of evaporation from human-made materials. Nearly half (49%) of air pollution comes from transportation, 28% from factories and power plants, and the remaining pollution from a variety of other sources.
Fossil Fuels
Fossil fuels are burned in most motor vehicles and power plants. These non-renewable resources are the power for nearly all manufacturing and other industries. Pure coal and petroleum can burn cleanly and emit only carbon dioxide and water, but most of the time these fossil fuels do not burn completely and the incomplete chemical reactions produce pollutants. Few sources of these fossil fuels are pure, so other pollutants are usually released. These pollutants include carbon monoxide, nitrogen dioxide, sulfur dioxide, and hydrocarbons.
In large car-dependent cities such as Los Angeles and Mexico City, 80% to 85% of air pollution comes from motor vehicles (Figure below). Ozone, carbon monoxide, and nitrous oxides come from vehicle exhaust.
Auto exhaust like this means that the fuels is not burning efficiently.
A few pollutants come primarily from power plants or industrial plants that burn coal or oil. Sulfur dioxide (SO2) is a major component of industrial air pollution that is released whenever coal and petroleum are burned. SO2 mixes with H2O in the air to produce sulfuric acid (H2SO4).
Mercury is released when coal and some types of wastes are burned. Mercury is emitted as a gas, but as it cools, it becomes a droplet. Mercury droplets eventually fall to the ground. If they fall into sediments, bacteria convert them to the most dangerous form of mercury: methyl mercury. Highly toxic, methyl mercury is one of the metal’s organic forms.
Biomass Burning
Fossil fuels are ancient plants and animals that have been converted into usable hydrocarbons. Burning plant and animal material directly also produces pollutants. Biomass is the total amount of living material found in an environment. The biomass of a rainforest is the amount of living material found in that rainforest.
The primary way biomass is burned is for slash-and-burn agriculture (Figure below). The rainforest is slashed down and then the waste is burned to clear the land for farming. Biomass from other biomes, such as the savannah, is also burned to clear farmland. The pollutants are much the same as from burning fossil fuels: CO2, carbon monoxide, methane, particulates, nitrous oxide, hydrocarbons, and organic and elemental carbon. Burning forests increases greenhouse gases in the atmosphere by releasing the CO2 stored in the biomass and also by removing the forest so that it cannot store CO2 in the future. As with all forms of air pollution, the smoke from biomass burning often spreads far and pollutants can plague neighboring states or countries.
A forest that has been slash-and-burned to make new farmland.
Particulates result when anything is burned. About 40% of the particulates that enter the atmosphere above the United States are from industry and about 17% are from vehicles. Particulates also occur naturally from volcanic eruptions or windblown dust. Like other pollutants, they travel all around the world on atmospheric currents.
Evaporation
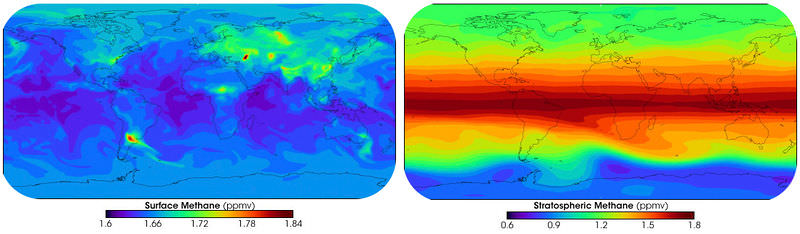
Volatile organic compounds (VOCs) enter the atmosphere by evaporation. VOCs evaporate from human-made substances, such as paint thinners, dry cleaning solvents, petroleum, wood preservatives, and other liquids. Naturally occurring VOCs evaporate off of pine and citrus trees. The atmosphere contains tens of thousands of different VOCs, nearly 100 of which are monitored. The most common is methane, a greenhouse gas (Figure below). Methane occurs naturally, but human agriculture is increasing the amount of methane in the atmosphere.
Methane forms when organic material decomposes in an oxygen-poor environment. In the top image, surface methane production is shown. Stratospheric methane concentrations in the bottom image show that methane is carried up into the stratosphere by the upward flow of air in the tropics.
Summary
- Most fossil fuels are dirty and release pollutants such as carbon monoxide, nitrogen dioxide, sulfur dioxide, and hydrocarbons.
- Burning plants and other biomass releases pollutants including carbon monoxide, methane, particulates, nitrous oxide, hydrocarbons, and organic and elemental carbon.
- Volatile organic compounds evaporate into the air and become pollutants.
Review
- What is slash-and-burn agriculture and what pollutants does it release?
- What are volatile organic compounds and why are they pollutants?
- Name a compound that occurs in the atmosphere naturally but is a pollutant in excess amounts due to human activities.