4.4: IV. Using Central Ideas about Light and Thermal Phenomena to Explain the Greenhouse Effect
- Page ID
- 15136
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)31
IV. Using Central Ideas about Light and Thermal Phenomena to Explain the Greenhouse Effect
Emily van Zee and Elizabeth Gire
The greenhouse effect is often mentioned in discussions about global climate. The name of this effect refers to an analogy between what happens when the Sun shines on the entire Earth and what happens when the Sun shines on a greenhouse in a garden here on Earth.
A. Considering what happens during the greenhouse effect in a garden greenhouse
A student noted that sometimes people have no idea what is happening in a greenhouse here on Earth:
I asked my sister if she knew anything about the greenhouse effect and she said nothing.So I asked if she ever heard of it before and she said no. Then I reminded her that greenhouses are used for gardens. She went ‘Oh, right’ but didn’t know what they did for the gardens…
Physics 111 Student, Spring 2016
Therefore it can be helpful to start this discussion with a focus on greenhouses in gardens here on Earth
Question 4.7 What is the greenhouse effect that occurs within a greenhouse in a garden?
- Figure 4.10 shows a photograph of a greenhouse. What do you know about greenhouses: How are they made? What is their purpose? Why do they work?

FIG. 4.10 A greenhouse in a garden by Steve Daniels CC BY-SA 2.0 Source: https://www.geograph.org.uk/of/4182114
To explore the greenhouse effect in a greenhouse in a garden, you will need:
- 2 identical clear glass or plastic containers,
- 2 thermometers that agree on the same number for room temperature or 2 digital temperature probes connected to a computer and calibrated so that they read the same temperature
- 2 rulers,
- 2 moist paper towels
- 2 identical lamps with identical bulbs or one lamp that can shine equally on both containers or access to a place to put the containers in the Sun)
- clear plastic wrap or glass cover for one of the containers.
- How could you use this equipment to explore what happens when an energy source (the Sun or a lamp) shines on an open versus a closed container?
- To model the greenhouse effect that occurs in garden greenhouses, prepare two clear glass or plastic containers in the same way:
- put two paper towels moistened in the same way in the bottom of each container
- check that two thermometers or two temperature probes connected to a computer give the same reading for room temperature
- place a ruler diagonally in each container with one end on the bottom and the other end resting on an edge of the container
- lay a thermometer on the ruler in each container so that the bulb of the thermometer is not resting on the bottom of the container but is supported at about a third of the height of the container, with the scale facing up so that the thermometer reading is visible (or place a digital thermometer on the ruler in each container)
- record the initial temperatures of the air in the containers; these should be the same
- At the top of your physics notebook page, record the Topic of this exploration. Under Before, draw a picture of the set up. What do you predict will happen to the temperatures of the containers after one container is covered and both are placed in the Sun or under identical lamps placed the same distance away from the containers? Why do you predict this will happen?
- Continue preparing to model the greenhouse effect:
- cover one of the containers with a clear glass plate or plastic wrap
- place the containers in the sun or place two identical lamps with identical bulbs so that they shine on the containers in the same way from the same distance away or place one lamp so it shines equally on the two containers
- monitor the temperatures every few minutes or so
- Under the During section of your physics notebook page, make a table recording the temperatures of the containers or draw or take a picture of the graph drawn by the computer connected to the temperature probes.
- Note any vocabulary that is new to you.
- Discuss your findings and formulate a relevant central idea. In the After section of the physics notebook page, report this central idea and the evidence on which it is based.
- Write a rationale that explains how the evidence supports this idea and why this is important
- Also reflect upon this exploration such as what connections can you make to other experiences? How might you use what you learned in your own classroom?
- What have you learned and what are you still wondering?
Write a summary of what you have learned about the greenhouse effect in a garden and explain why a greenhouse in a garden gets warm when light from the Sun shines upon it.
Complete documenting your exploration on your physics notebook page and writing a summary before looking at an example of student work about exploring the greenhouse effect in garden greenhouses.
1. Example of student work about exploring the greenhouse effect in garden greenhouses
Greenhouse effect. (Figure 4.11) is a sketch of the experiment done in class.
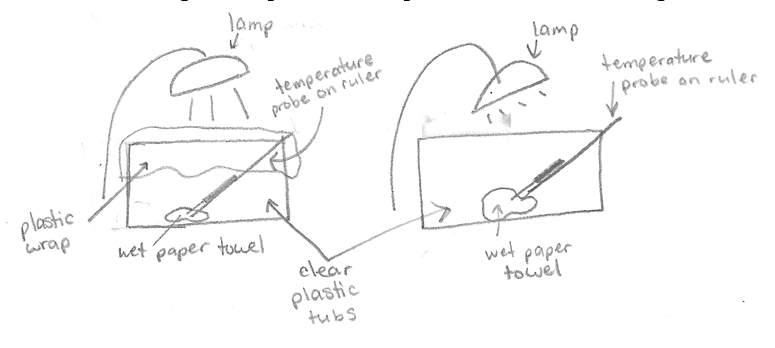
The student labeled two lamps shining on two clear plastic tubs. Both tubs had wet paper towels in the bottom and temperature probes on rulers leaning on one edge of the tub, with the ruler resting on the bottom. One tub had plastic wrap covering the top of the tub.
Two plastic tubs were set up under lamps with a wet paper towel in each. Both tubs also had a temperature probe attached to a ruler so we could track any changes in temperature that may have occurred. One tub had plastic wrap covering the opening, and the other had no covering. We predicted what would happen, and many of us thought that the one with plastic would be warmer, since heat could not escape as easily as the tub without plastic wrap. At the beginning of this experiment both temperatures were about 22°C. Later in the lab we returned to these tubs to see that the tub with plastic wrap had condensation on the plastic, and was much warmer than the other tub. At the end of this experiment, the tub without plastic was 27.9° and the tub with the plastic wrap was 35.7°C. Both tubs had an increase in temperature as a result of heat from the lamp. However, with the plastic wrap blocking heat (energy) from leaving the tub, it was much hotter than the tub without a blocked exit. Like this model, if greenhouse gasses block energy from leaving the Earth, then Earth’s temperature will drastically increase.
Physics student, Spring 2016
The greenhouse effect in this model of greenhouses in gardens involves an enclosed container getting warmer when more energy from the light source enters than leaves the container. The warmed air in the open container can circulate outside the container so the open container does not warm as much as the covered container.
Enclosed greenhouses in gardens prevent warmed air from circulating with cooler air outside. In addition, the garden greenhouse may be made out of glass that transmits visible light but not infrared light. Although the plants use some of the energy they absorb to grow, they also emit infrared radiation as they warm, as do the other contents of the greenhouse such as the soil, tables, and tools. If the infrared radiation cannot travel out through the glass and remains within the greenhouse, the temperature of the contents and the air increases. This mechanism differs from what happens when light from the Sun shines on the entire Earth, but the overall effect, an increase in temperature because of energy that does not leave the system, is the same.
If more energy enters than leaves, a system warms up. Within a garden greenhouse, the owner can use vents to modulate the energy flow in and out if the greenhouse gets too hot (see, for example, https://www.advancingalternatives.com/blog/getting-started-greenhouse-ventilation-systems/. With a greenhouse effect operating within the entire Earth, however, what can the Earth’s “owners” do if the Earth gets too hot? That is the issue that concerns scientists and others convinced on the basis of evidence that the Earth is warming up now much more rapidly than in the past.
B. Considering what happens during the greenhouse effect on a global scale
Question 4.8 What is the greenhouse effect in the context of the entire Earth?
To consider the greenhouse effect in the context of the entire Earth, each group will need: a large white board as well as a white board marker and eraser for each student.- With your group members, talk about the greenhouse effect on a global scale.
- On a large white board, draw a diagram to represent your group’s initial ideas about what is happening to the energy that enters the Earth’s system when light from the Sun shines on the Earth:
- Where does this energy go?
- What changes does it undergo while on Earth?
- How does it leave the Earth?
- Plan and practice briefly what each group member will say when presenting your group’s diagram to the whole group.
- Share your ideas and their representation on a whiteboard with the whole group:
- What patterns do you notice in these presentations?
- How are the groups’ ideas similar? How are they different?
- How do they help you think about the greenhouse effect on the Earth?
1. Examples of students’ initial diagrams about the greenhouse effect
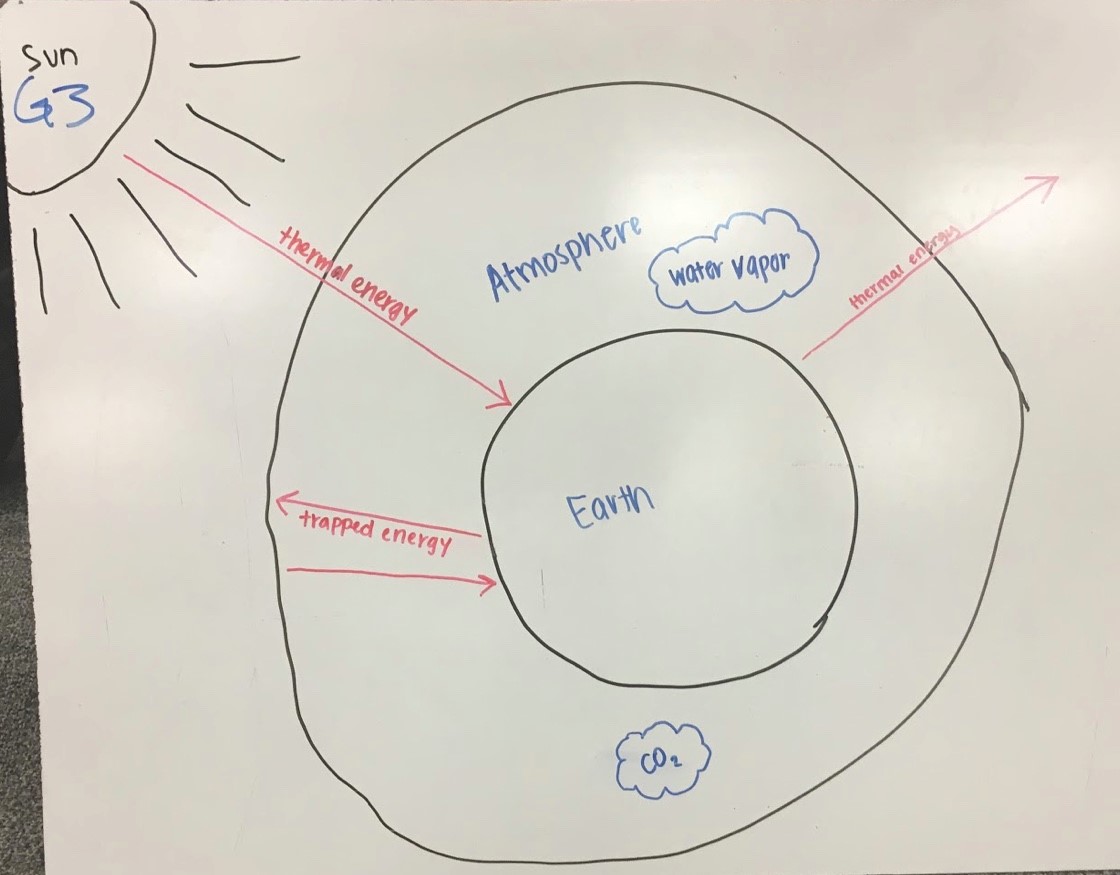
As shown in Fig. 4.12, Group 3’s diagram portrays the basic idea of the greenhouse effect, that thermal energy travels in rays from the Sun to the surface of the Earth and some of this thermal energy travels back out from the surface through the atmosphere to space. Some energy travels from the surface into the atmosphere but is trapped and returns back to the surface. The atmosphere includes two gases, water vapor and carbon dioxide that are relevant to this process.
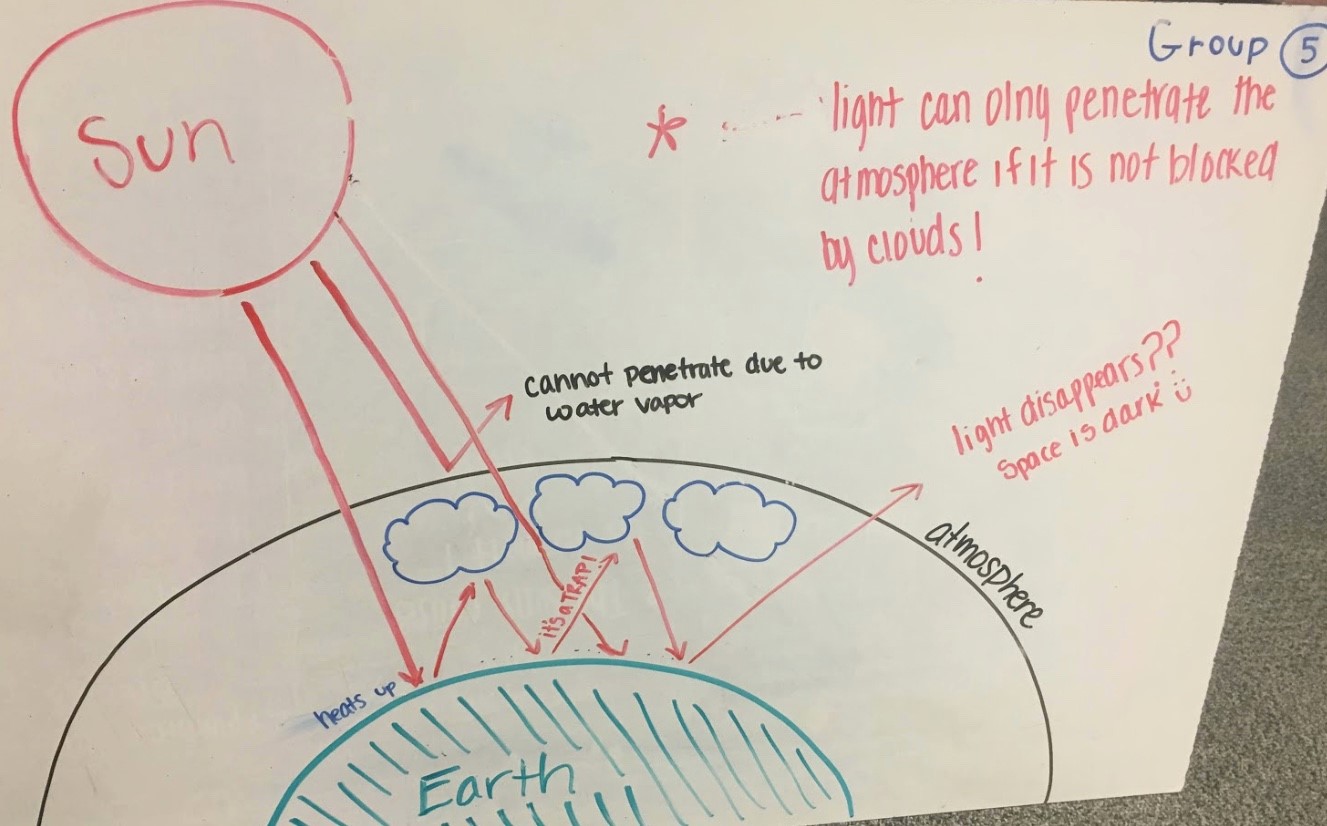
As shown in Fig. 4.13, Group 5’s diagram portrays the basic idea of the greenhouse effect with some details. These include that rays from the Sunheat up the surface of the Earth; that some rays cannot penetrate the atmosphere due to water vapor; that some rays bounce back and forth between the Earth’s surface and clouds in the atmosphere (It’s a TRAP!), and some rays escape out to space, which raises an interesting question, does light disappear?? in space because Space is dark. A red star draws attention to the statement light can only penetrate the atmosphere if it is not blocked by clouds!
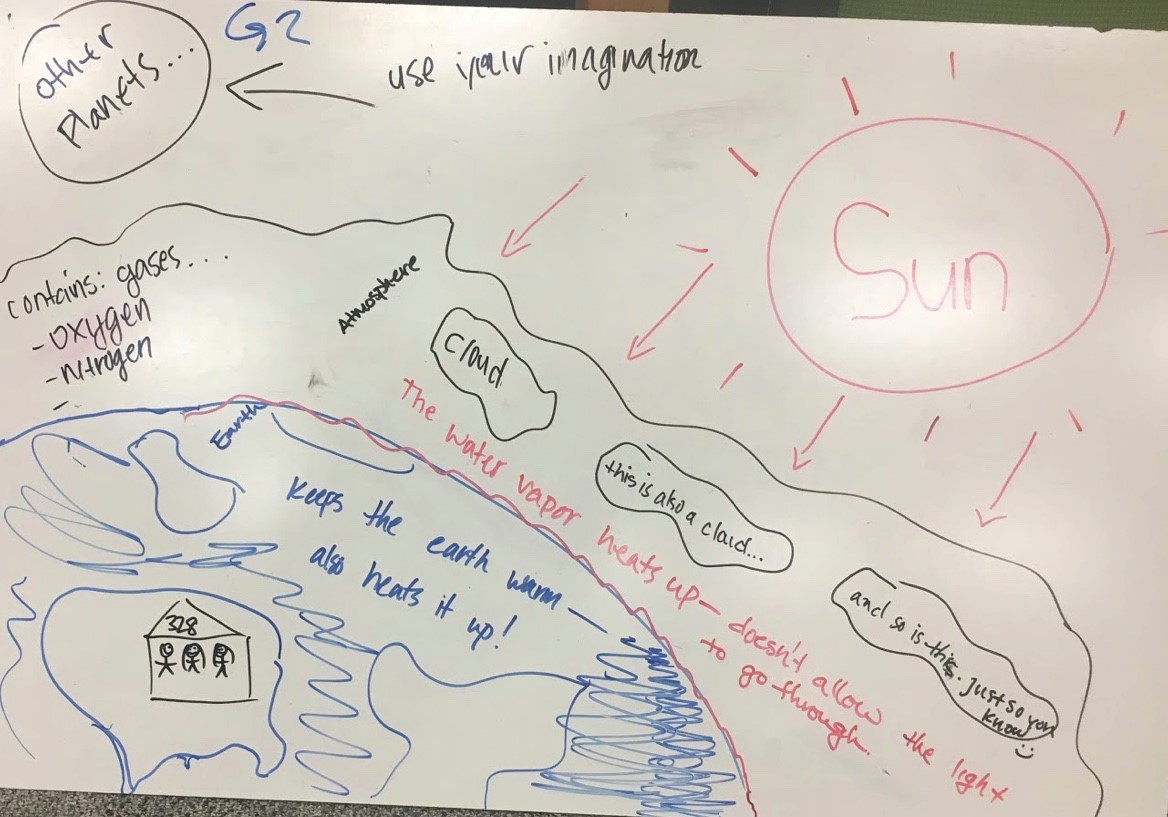
As shown in Fig. 4.14, Group 2’s diagram portrays the basic idea of the greenhouse effect with some additional details. The Sun sends rays in many directions, including toward Earth. Implied is that some of these rays get through the atmosphere and interact with the surface, which keeps the earth warm – also heats it up! The surface includes our classroom, Room 328, within a building within our country. Three clouds represent what happens in the atmosphere: a cloud, this is also a cloud…and so is this. Just so you know . The water vapor heats up – doesn’t allow the light to go through. The atmosphere contains : gases…oxygen and nitrogen. These students recognized that the Sun affects other planets and they suggest use your imagination to consider the Sun’s effect there.
The students moved their chairs to form a circle and each group presented their whiteboard to the others. These examples demonstrate that the students already had useful knowledge about the importance of the Sun and the atmosphere, about some of the relevant processes, and about their effects. Many of the details needed elaboration and/or refinement but the small groups were able to make reasonable first attempts at creating these complex diagrams. The actions of talking with one another in the small groups, creating their group’s initial greenhouse effect diagrams, and sharing these ideas and diagrams through the circle conversation set the context for studying a controversial and complex topic in a respectful way.
2. Greenhouse effect diagram provided by the Intergovernmental Panel on Climate Change
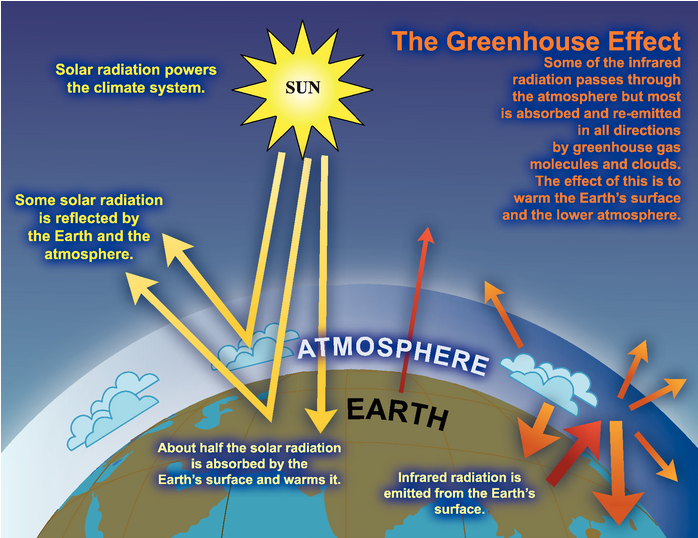
The Intergovernmental Panel on Climate Change (IPCC, www.ipcc.ch) is an organization with 195 member countries through which scientists work together to collect and analyze information about climate change from studies all over the world. Figure 4.15 presents a diagram prepared by this organization to represent what happens to the energy that radiates to the Earth from the Sun.
https://www.ipcc.ch/report/ar4/wg1/historical-overview-of-climate-change-science/
- To interpret this diagram, tell the story of what happens to the energy radiated from the Sun as it moves through the Earth’s climate system:
- What do each of the three yellow rays represent?
- What does the narrow reddish ray represent?
- What does the thick reddish ray represent?
- What does each of the orange rays represent?
- Put “greenhouse effect diagram” in your computer browser and view the many versions available for representing the greenhouse effect. Select one or make your own and write your own interpretation of a diagram presenting the greenhouse effect.
3. Example of student’s written work about the greenhouse effect on the entire Earth
I went to the Internet to learn more about the process and the flow of energy surrounding the greenhouse effect. I came upon this diagram:
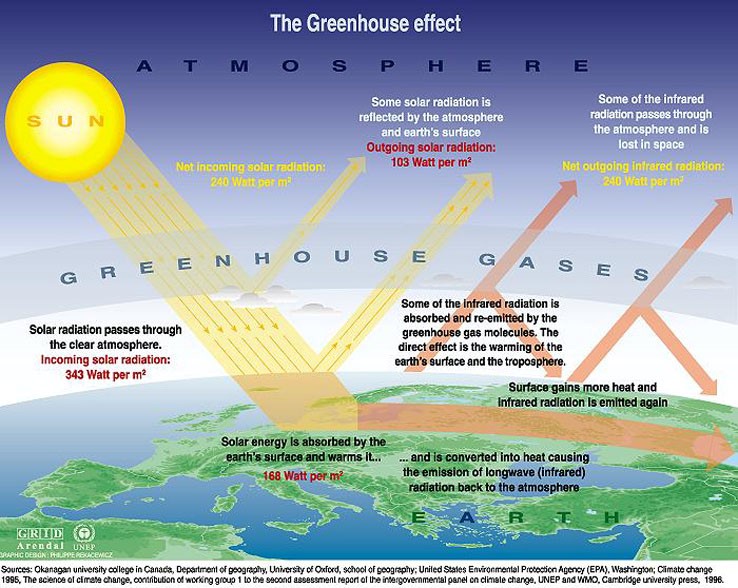
I used this diagram to help create my own diagram, because I learn best though my own creations. I decided to make a diagram that had steps to show how the radiation from the sun goes through a course to heat up the earth:
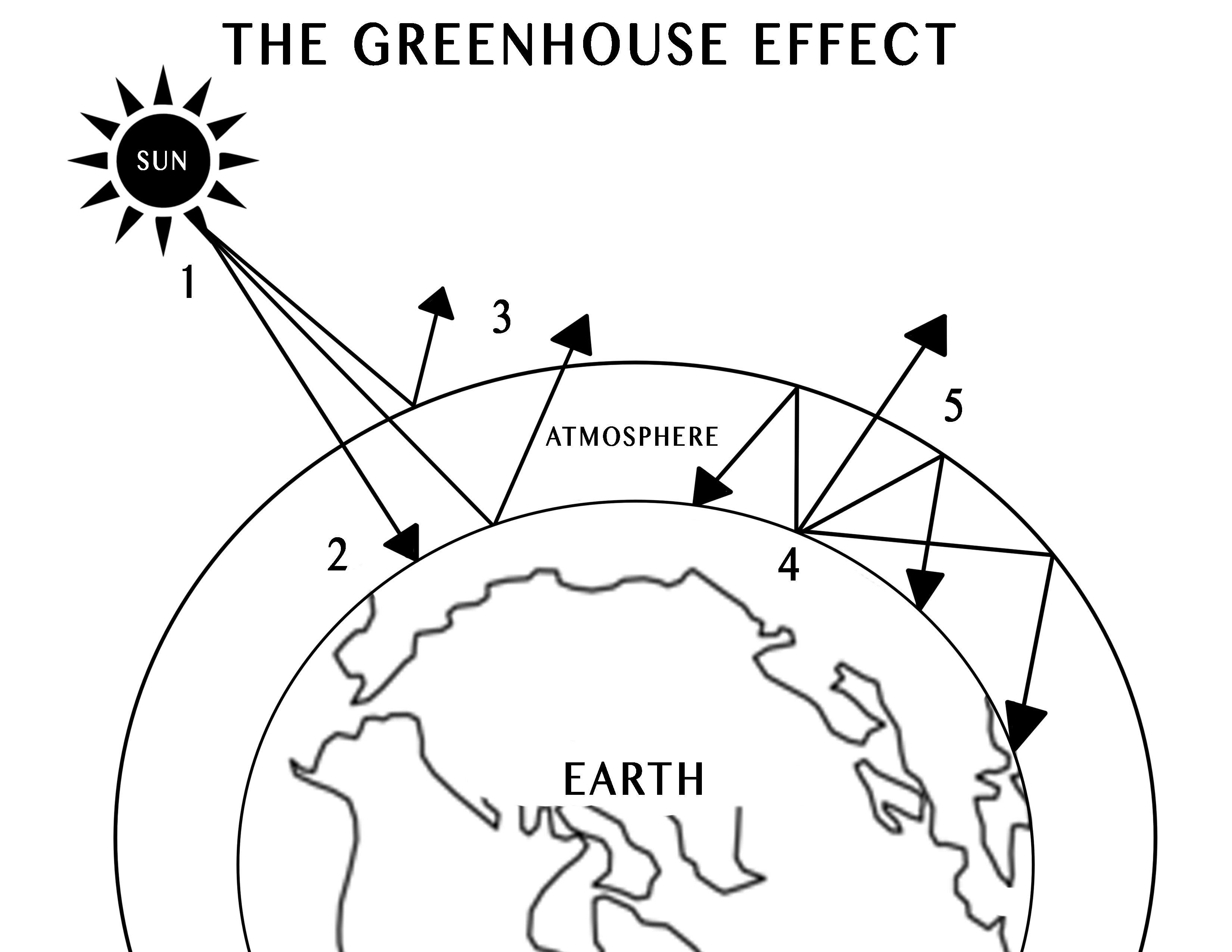
-
-
- Solar radiation is emitted from the sun, headed toward Earth.
- Most of the sun’s radiation is absorbed by the earth’s surface, thus warming the earth.
- Some of the solar radiation is reflected back out to space by the earth surface and atmosphere.
- As the solar radiation heats up the earth’s surface and lower atmosphere, the radiation is converted into thermal energy called infrared radiation.
- Some of the infrared radiation escapes Earth’s atmosphere, but most of it is absorbed and re-emitted to the earth by the greenhouse gasses in the atmosphere, warming the surface even more.
-
The earth should be able to keep a proper balance of energy in and energy out in order to keep the surface temperature stable for our current ecosystems; however this is no longer true. The earth’s “energy budget” has been disrupted by the presence of more than the natural amount of greenhouse gases in our atmosphere. Deforestation, excessive use of fossil fuels, and massive beef consumption all are factors that contribute to excess greenhouse gasses such as carbon dioxide and methane. Infrared rays have a hard time passing though these gases in the atmosphere, trapping more from escaping. This means that the earth is receiving the same amount of energy from the sun as it always had, but it is unable to release a balanced amount back out to space, throwing off the energy budget. Unfortunately, while there are things we as humans can do to aid in this issue, many people are not willing to make the necessary changes in order to save the planet.
Physics Student, Fall 2016
4. Nuances about the greenhouse effect and the Earth’s energy budget
Some students are curious about what it means for the infrared radiation to be “trapped” by the green house gases. Some have questions about the details involved in what happens to the energy entering and leaving the Earth’s system. This section provides additional information for those interested.
(a) Mechanism that underlies the statement that energy is “trapped” by greenhouse gases. Discussions of the greenhouse effect often refer to energy being “trapped” by greenhouse gases in the atmosphere. What are greenhouse gases and what does being “trapped” mean in this context?
The Earth’s atmosphere is composed of a mixture of gaseous molecules (see, for example, http://climate.ncsu.edu/edu/Atmosphere). Most of the atmosphere consists of nitrogen molecules (N2) and oxygen molecules (O2). These molecules transmit the sunlight shining through them. Greenhouse gases, however, are molecules that interact with infrared radiation from the Sun as well as with infrared radiation emitted from the surface of the Earth. The major greenhouse gases are:
-
-
- water vapor, formed by two atoms of hydrogen bonded to one atom of oxygen, H2O
- carbon dioxide, formed by one atom of carbon and two atoms of oxygen, CO2
- methane, formed by one atom of carbon and four atoms of hydrogen, CH4.
-
The greenhouse gas molecules in the atmosphere “trap” energy by absorbing infrared radiation and then emitting infrared radiation in all directions, including back toward the surface of the Earth. These gases absorb and emit infrared radiation by vibrating in complex ways. See, http://energyeducation.ca/encyclopedia/Infrared_radiation, for example, and https://www.youtube.com/watch?v=AauIOanNaWk for forms of vibration in carbon dioxide molecules. A diagram of such vibrations is shown in Fig. 4.18.
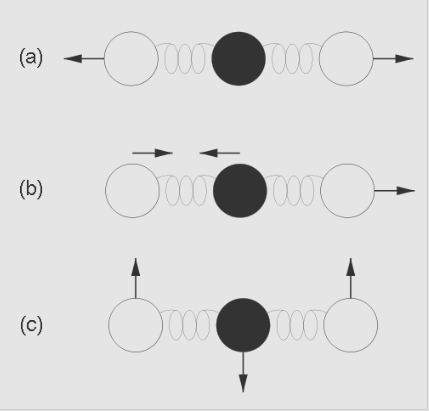
Credit: Martin C. Doege, Windows to the Universe®, © 2007 National Earth Science Teachers Association, https://www.windows2universe.org/earth/climate/greenhouse_effect_gases.html CC 3.0
The black dot represents a carbon atom, the two clear dots represent the oxygen atoms and the coils of springs represent the chemical bonds holding these atoms together as one molecule. The vibrations may involve (a) the two oxygen atoms both moving away from the carbon atom, (b) one oxygen atom and the carbon atom moving toward each other while the other oxygen atom moves away, or (c) the carbon atom moving one way perpendicular to the bonds while the two oxygen atoms move in the opposite direction.
This mechanism of the greenhouse gas molecules absorbing energy by vibration differs from the mechanism creating the greenhouse effect in greenhouses on earth. Green houses made out of glass panes that do not transmit infrared radiation warm up when incoming visible light is transmitted through the glass panes, absorbed by the contents of a greenhouse, emitted as infrared radiation, but not transmitted back out through the glass panes. Also, the greenhouse effect can occur simply by enclosing a container as in the exploration described in Question 4.7. However, the effects are the same in that the temperature of a system increases if more energy enters a system than leaves it.
(b) Details about what happens to energy entering and leaving the Earth’s system. Figure 4.19 presents a more detailed analysis of what would happen to the energy radiated from the Sun to the Earth during the greenhouse effect process if the Earth’s energy budget were balanced. To be in balance:
-
-
- the incoming energy at the edge of the atmosphere should equal the outgoing energy at the edge of the atmosphere;
- the incoming energy within the atmosphere should equal the outgoing energy within the atmosphere and
- the incoming energy absorbed by the surface of the Earth should equal the outgoing energy at the surface.
-
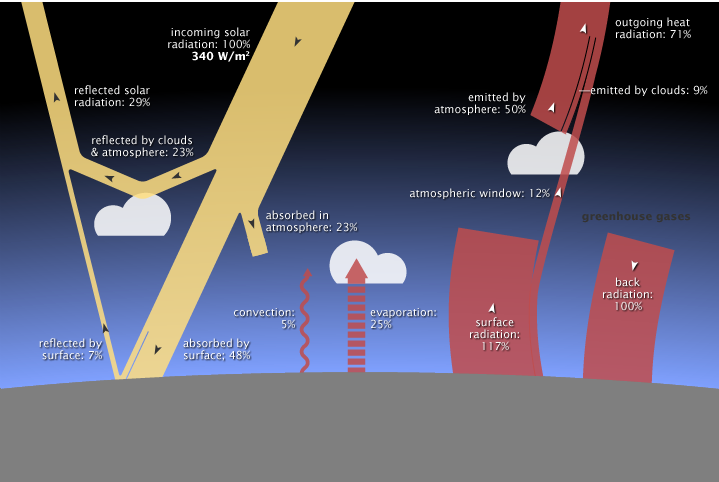
https://earthobservatory.nasa.gov/Features/EnergyBalance/page6.php
-
-
- What happens at the edge of the atmosphere if the Earth’s system is in balance? The thick yellow ray represents the incoming energy from the Sun (100%). The thinner yellow ray at the upper left corner of the diagram represents that 29% of that energy would be reflected back out into space from clouds, the atmosphere, and the surface. The somewhat thick red ray near the top right of the diagram represents that 71% of that energy would pass back out of the Earth’s atmosphere into space. If the Earth’s energy budget were in balance, the total incoming radiation would be balanced by the total outgoing radiation after reflection by the clouds, atmosphere and surface as well as after various absorption and emission processes within the atmosphere.
- What happens within the atmosphere if the Earth’s system is in balance? The thin yellow branch of the incoming ray that points into the atmosphere represents the 23% of the incoming energy absorbed by the atmosphere. Also entering the atmosphere are three sources from the surface: convection as in the sunny day at the beach (5%), evaporation (25%), and surface radiation (117%) for a total of 170%. Leaving the atmosphere is energy emitted by the atmosphere (50%), emitted by clouds (9%), and energy from the surface that gets through the “atmospheric window” directly out to space (12%) for a total of 71% and also energy emitted from greenhouse gases back in the direction of the surface (100%) for a total of 171%, which is balanced (the extra 1% probably due to rounding).
- What happens at the surface of the Earth if the Earth’s system is in balance? Incoming energy absorbed by the surface would be transferred to the atmosphere by several processes: The thin waving red ray, pointing upward, represents transfer of the incoming energy through convection (5%) in ways similar to those described in explaining sea breezes in Unit 3. Air warmed by the surface by conduction, expands, rises into the cool upper atmosphere, and cools as energy flows from the warm air into the cooler surrounding atmosphere. The red ray made out of horizontal segments, pointing upward, represents transfer of energy from the surface to the atmosphere through evaporation (25%) such as from puddles, streams, oceans and through transpiration by plants. When moist air is warmed, rises, and cools, the gaseous water releases energy as it condenses into droplets, forming clouds. The thick red ray with the arrow pointing upward represents the transfer of energy from the surface to the atmosphere when the surface emits infrared radiation (117%) at a rate determined by its temperature. The total energy from the surface to the atmosphere would be 148%. Some gases such as water vapor, carbon dioxide, and methane absorb most of the infrared radiation and re-emit it in all directions, including back down toward the surface of the Earth (100%), represented by the red ray with the arrow pointing downward. There would be a net sum of the energy emitted from the surface that would equal the incoming energy that is absorbed by the surface. Also some of the energy from the sun shines directly on the surface (48%) represented by the yellow ray pointed toward the surface. The energy reaching the surface (148%) would balance the energy leaving the surface (148%).
-
As the amount of water vapor, carbon dioxide, and methane gases in the atmosphere increases, however, more infrared radiation will be absorbed, emitted in all directions, including back toward the surface, with more and more energy staying in the Earth’s system, increasing the global temperature of the Earth. For more information about the Earth’s energy budget, search on the Internet for “Earth’s energy budget” and view, for example, a series of pages (4-7) at https://earthobservatory.nasa.gov/Features/EnergyBalance/page4.php
To deepen understanding about the influence of light and thermal phenomena on global climate read some students’ reflections about engaging a friend in learning about the greenhouse effect.
5. Examples of student work reflecting upon engaging a friend or family member in learning about the greenhouse effect
The students explored websites that discuss the greenhouse effect and selected one or more to use in engaging a friend or family member in learning about the greenhouse effect. One student wrote:
I chose to explore the greenhouse effect with my two friends. Prior to exploring the website, they told me that they knew that the greenhouse effect was related to “gases getting trapped in the atmosphere and heating it up.” The website, https://energyeducation.ca/encyclopedia/Greenhouse_effect is provided by Energy Education Canada. Both of them said that is was very helpful to observe a diagram depicting the greenhouse effect, which aided their understanding of the readings provided by the website. One of my friends said that the thing that hindered his understanding was that the arrow accompanying the text that reads, “Infrared radiation is emitted by the Earth’s surface,” emerges from water, rather than from land or a combination of land and water, which led him to believe that the diagram was indicating that only water emits infrared radiation.
Both of them asked me what infrared radiation is, and I explained to them that infrared emits energy in the form of heat, as well as that the color of an object when observed through night vision goggles indicates how much infrared radiation it emits, with objects that appear red emitting a lot of infrared radiation, and objects that appear blue emitting much less infrared radiation. Neither of them had prior knowledge of infrared radiation, and they stated that this was the most interesting thing they learned from the website. Additionally, neither of them had ever heard the term “enhanced greenhouse effect,” which is what is referred to in discussions of the greenhouse effect and climate change.
When I asked them how they felt about the potential consequences of climate change, including rising sea levels, they both wondered if climate change will drastically affect them personally in their lifetime. Additionally, they wondered how much of the greenhouse effect is due to livestock, which sparked an interesting discussion about how many cows and chickens are on the earth, as well as how the raising and consuming of animal products contributes to climate change. This experience taught me that sometimes a conversation may not go the way you expect it to, but it can still lead to beneficial discussion and learning. I was not expecting to discuss livestock with them, but they were very interested in the various layers of climate change, and I felt that they gained a lot from the website and grew in their curiosity throughout our discussion.
Both of my friends engaged in the NGSS Science practice of obtaining, evaluating, and communicating information during this exploration. Not only did they obtain information from the website that I showed them, but when our discussion shifted toward the effect of livestock farming on climate change, they both began to research articles on the topic, obtained knowledge from them, and shared that knowledge to enhance our discussion. They also engaged in the NGSS crosscutting concept of systems and system models, as the diagram from the website helped them to envision the system of the greenhouse effect and see how it works.
Physics student, Winter 2018
Another student chose to talk about the greenhouse effect with her father:
So I did this assignment with my father. I had a feeling it would not go well but I wanted to show him evidence of climate change. I first started by asking him what he knew about the greenhouse effect. He said “It causes global warming, climate change, and has something to do with too many cows giving off ethanol into the air.” I then decided to show him the NASA website https://climate.nasa.gov/causes/ because it was my favorite. We looked over the website. It was easy to navigate the website and the pictures were really helpful. The pictures helped the most and the website even had before and after photos of bad events around the world. We talked about the future effects of global warming but he did not ask any questions. After we finished exploring the website I explained the model diagram of the greenhouse effect again and explained that humans are the real cause of this. I then asked what my father thought about global warming. He said “the planet has always been changing and it always will.” So after showing all this proof to my father he then says “it’s propaganda.” My father just nodded while looking at the website.
I think my father did not have any questions because he is closed off and does not want to hear that humans are causing the Earth to heat up and it could harm/ is harming the environment. I went into more detail about the greenhouse gasses being the real cause and that human activity is the cause but he just wasn’t interested. Through this experience I think I have learned that it is hard to teach topics that people already think they know or feel strongly one way about. No matter how much evidence is shown. It is discouraging to think about how many people are out there, unable to face the facts that global warming is real. The older I get, the more I realize a lot of people do not listen to science and just believe what they want to. For example, learning about vaccines. It does not matter how much we show the scientific evidence of the safety and success of vaccines, people still believe they cause autism and make people sick.
The crosscutting concept of cause and effect is strongly shown in the topic of greenhouse gasses because we are showing that human activity is causing an effect on the Earth, causing it to warm up. One NGSS practice used was modeling. We used the model given on the website to help explain the greenhouse effect.
Physics student, Winter 2018
These students had very different experiences, with a welcome sharing of thoughts and additional learning for all concerned in the first and with a reluctant listener not yet open to alternative points of view in the second. Afterward, the second student raised in class the question of what to do. This is a difficult question to which there are no easy answers. To what extent is one willing to risk personal relationships in discussing controversial topics? How can one convey information based on evidence effectively? How can one keep one’s own spirits up in the face of such discouraging encounters? Our hope is that the small group activities and whole group discussions in class will at least make possible more positive experiences as well as increase information getting to the reluctant listeners whose friends or relatives choose to risk the conversation.

