2.2: Diffusion
- Page ID
- 8356
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
What will eventually happen to these dyes?
They will all blend together. The dyes will move through the water until an even distribution is achieved. The process of moving from areas of high amounts to areas of low amounts is called diffusion.
Passive Transport
Probably the most important feature of a cell’s phospholipid membranes is that they are selectively permeable or semipermeable. A membrane that is selectively permeable has control over what molecules or ions can enter or leave the cell, as shown in the Figure below. The permeability of a membrane is dependent on the organization and characteristics of the membrane lipids and proteins. In this way, cell membranes help maintain a state of homeostasis within cells (and tissues, organs, and organ systems) so that an organism can stay alive and healthy.
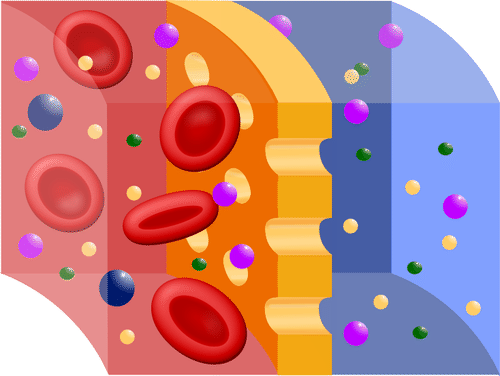 A selectively permeable membrane allows certain molecules through, but not others.
A selectively permeable membrane allows certain molecules through, but not others.Transport Across Membranes
The molecular make-up of the phospholipid bilayer limits the types of molecules that can pass through it. For example, hydrophobic (water-hating) molecules, such as carbon dioxide (CO2) and oxygen (O2), can easily pass through the lipid bilayer, but ions such as calcium (Ca2+) and polar molecules such as water (H2O) cannot. The hydrophobic interior of the phospholipid bilayer does not allow ions or polar molecules through because these molecules are hydrophilic, or water loving. In addition, large molecules such as sugars and proteins are too big to pass through the bilayer. Transport proteins within the membrane allow these molecules to pass through the membrane, and into or out of the cell. This way, polar molecules avoid contact with the nonpolar interior of the membrane, and large molecules are moved through large pores.
Every cell is contained within a membrane punctuated with transport proteins that act as channels or pumps to let in or force out certain molecules. The purpose of the transport proteins is to protect the cell's internal environment and to keep its balance of salts, nutrients, and proteins within a range that keeps the cell and the organism alive.
There are three main ways that molecules can pass through a phospholipid membrane. The first way requires no energy input by the cell and is called passive transport. The second way requires that the cell uses energy to pull in or pump out certain molecules and ions and is called active transport. The third way is through vesicle transport, in which large molecules are moved across the membrane in bubble-like sacks that are made from pieces of the membrane.
Passive transport is a way that small molecules or ions move across the cell membrane without input of energy by the cell. The three main kinds of passive transport are diffusion, osmosis, and facilitated diffusion.
Diffusion
Diffusion is the movement of molecules from an area of high concentration of the molecules to an area with a lower concentration. The difference in the concentrations of the molecules in the two areas is called the concentration gradient. Diffusion will continue until this gradient has been eliminated. Since diffusion moves materials from an area of higher concentration to the lower, it is described as moving solutes "down the concentration gradient." The end result of diffusion is an equal concentration, or equilibrium, of molecules on both sides of the membrane.
If a molecule can pass freely through a cell membrane, it will cross the membrane by diffusion (see the Figure below).
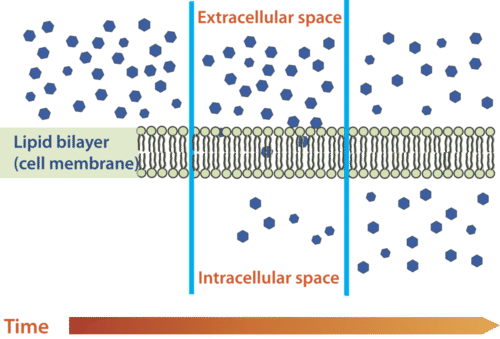 Molecules move from an area of high concentration to an area of lower concentration until an equilibrium is met. The molecules continue to cross the membrane at equilibrium, but at equal rates in both directions.
Molecules move from an area of high concentration to an area of lower concentration until an equilibrium is met. The molecules continue to cross the membrane at equilibrium, but at equal rates in both directions.Summary
- The cell membrane is selectively permeable, allowing only certain substances to pass through.
- Passive transport is a way that small molecules or ions move across the cell membrane without input of energy by the cell. The three main kinds of passive transport are diffusion, osmosis, and facilitated diffusion.
- Diffusion is the movement of molecules from an area of high concentration of the molecules to an area with a lower concentration.
Review
- Define semipermeable.
- What is diffusion?
- What is a concentration gradient?
- What is meant by passive transport?
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Julo (Wikimedia);Oak Ridge National Laboratory;Hana Zavadska, based on image by Mariana Ruiz Villarreal (commons.wikimedia.org/wiki/Fi...embrane-en.svg) Source: commons.wikimedia.org/wiki/File:BloodPressure.jpg ; commons.wikimedia.org/wiki/File:Fatmouse.jpg ; CK-12 Foundation License: CC BY-NC |
 |
[Figure 2] | Credit: User:Pidalka44/Wikimedia Commons Source: commons.wikimedia.org/wiki/File:Semipermeable_membrane.png License: Public Domain |
 |
[Figure 3] | Credit: Hana Zavadska, based on image by Mariana Ruiz Villarreal (LadyofHats) (commons.wikimedia.org/wiki/Fi...embrane-en.svg);User:Pidalka44/Wikimedia Commons;LadyofHats Source: CK-12 Foundation ; commons.wikimedia.org/wiki/File:Semipermeable_membrane.png License: CC BY-NC 3.0; Public Domain |

