2.6: Sodium-Potassium Pump
- Page ID
- 8360
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
What is this incredible object?
Would it surprise you to learn that it is a human cell? The image represents an active human nerve cell. How nerve cells function will be the focus of another concept. However, active transport processes play a significant role in the function of these cells. Specifically, it is the sodium-potassium pump that is active in the axons of these nerve cells.
The Sodium-Potassium Pump
Active transport is the energy-requiring process of pumping molecules and ions across membranes "uphill" - against a concentration gradient. To move these molecules against their concentration gradient, a carrier protein is needed. Carrier proteins can work with a concentration gradient (during passive transport), but some carrier proteins can move solutes against the concentration gradient (from low concentration to high concentration), with an input of energy. In active transport, as carrier proteins are used to move materials against their concentration gradient, these proteins are known as pumps. As in other types of cellular activities, ATP supplies the energy for most active transport. One way ATP powers active transport is by transferring a phosphate group directly to a carrier protein. This may cause the carrier protein to change its shape, which moves the molecule or ion to the other side of the membrane. An example of this type of active transport system, as shown in the Figure below, is the sodium-potassium pump, which exchanges sodium ions for potassium ions across the plasma membrane of animal cells.
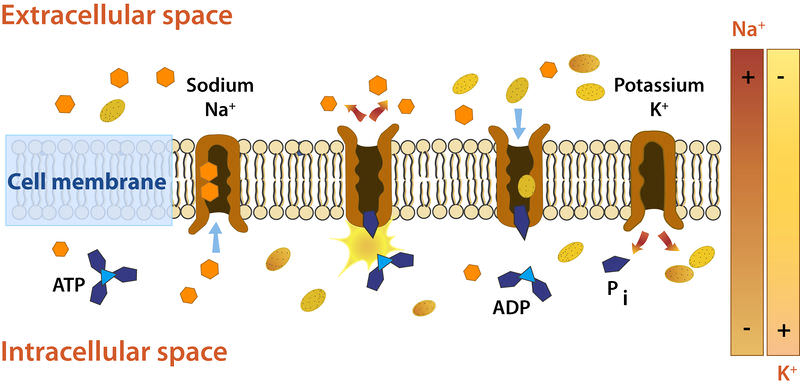 The sodium-potassium pump system moves sodium and potassium ions against large concentration gradients. It moves two potassium ions into the cell where potassium levels are high, and pumps three sodium ions out of the cell and into the extracellular fluid.
The sodium-potassium pump system moves sodium and potassium ions against large concentration gradients. It moves two potassium ions into the cell where potassium levels are high, and pumps three sodium ions out of the cell and into the extracellular fluid.As is shown in the Figure above, three sodium ions bind with the protein pump inside the cell. The carrier protein then gets energy from ATP and changes shape. In doing so, it pumps the three sodium ions out of the cell. At that point, two potassium ions from outside the cell bind to the protein pump. The potassium ions are then transported into the cell, and the process repeats. The sodium-potassium pump is found in the plasma membrane of almost every human cell and is common to all cellular life. It helps maintain cell potential and regulates cellular volume.
The Electrochemical Gradient
The active transport of ions across the membrane causes an electrical gradient to build up across the plasma membrane. The number of positively charged ions outside the cell is greater than the number of positively charged ions in the cytosol. This results in a relatively negative charge on the inside of the membrane, and a positive charge on the outside. This difference in charges causes a voltage across the membrane. Voltage is electrical potential energy that is caused by a separation of opposite charges, in this case across the membrane. The voltage across a membrane is called membrane potential. Membrane potential is very important for the conduction of electrical impulses along nerve cells.
Because the inside of the cell is negative compared to outside the cell, the membrane potential favors the movement of positively charged ions (cations) into the cell, and the movement of negative ions (anions) out of the cell. So, there are two forces that drive the diffusion of ions across the plasma membrane—a chemical force (the ions' concentration gradient), and an electrical force (the effect of the membrane potential on the ions’ movement). These two forces working together are called an electrochemical gradient, and will be discussed in detail in "Nerve Cells" and "Nerve Impulses" concepts.
Summary
- Active transport is the energy-requiring process of pumping molecules and ions across membranes against a concentration gradient.
- The sodium-potassium pump is an active transport pump that exchanges sodium ions for potassium ions.
Review
- What is active transport?
- What type of protein is involved in active transport?
- Describe how the sodium-potassium pump functions.
- What is the electrochemical gradient?
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Mariana Ruiz Villarreal (User:LadyofHats) Source: commons.wikimedia.org/wiki/File:Scheme_sodium-potassium_pump-en.svg License: Public Domain |
 |
[Figure 2] | Credit: Mariana Ruiz Villarreal (User:LadyofHats) Source: commons.wikimedia.org/wiki/File:Scheme_sodium-potassium_pump-en.svg License: Public Domain |

