12.22: Carbon Cycle
- Page ID
- 14464
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Why is Earth getting warmer?
What happens if carbon is not removed from the atmosphere? The excess carbon dioxide in the atmosphere is contributing to a global rise in Earth’s temperature, known as global warming. Where does this carbon dioxide come from? Burning gas to power our cars and burning coal to generate electricity are two main sources of the excess carbon dioxide.
The Carbon Cycle
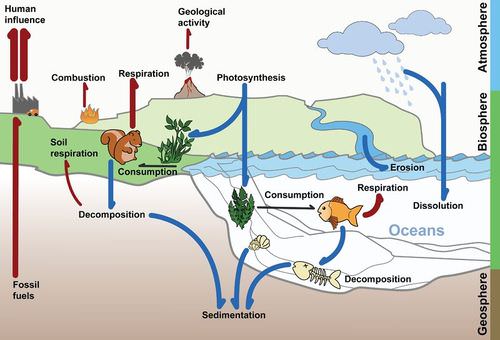
Carbon is one of the most common elements found in living organisms. Chains of carbon molecules form the backbones of many organic molecules, such as carbohydrates, proteins, and lipids. Carbon is constantly cycling between living organisms and the atmosphere (Figure below). The cycling of carbon occurs through the carbon cycle.
Living organisms cannot make their own carbon, so how is carbon incorporated into living organisms? In the atmosphere, carbon is in the form of carbon dioxide gas (CO2). Recall that plants and other producers capture the carbon dioxide and convert it to glucose (C6H12O6) through the process of photosynthesis. Then as animals eat plants or other animals, they gain the carbon from those organisms.
The chemical equation of photosynthesis is 6CO2 + 6H2O → C6H12O6+ 6O2.
How does this carbon in living things end up back in the atmosphere? Remember that we breathe out carbon dioxide. This carbon dioxide is generated through the process of cellular respiration, which has the reverse chemical reaction as photosynthesis. That means when our cells burn food (glucose) for energy, carbon dioxide is released. We, like all animals, exhale this carbon dioxide and return it back to the atmosphere. Also, carbon is released to the atmosphere as an organism dies and decomposes.
Cellular respiration and photosynthesis can be described as a cycle, as one uses carbon dioxide (and water) and makes oxygen (and glucose), and the other uses oxygen (and glucose) and makes carbon dioxide (and water).
Formation of Fossil Fuels
Millions of years ago, there were so many dead plants and animals that they could not completely decompose before they were buried. They were covered over by soil or sand, tar or ice. These dead plants and animals are organic matter made out of cells full of carbon-containing organic compounds (carbohydrates, lipids, proteins and nucleic acids). What happened to all this carbon? When organic matter is under pressure for millions of years, it forms fossil fuels. Fossil fuels are coal, oil, and natural gas.
When humans dig up and use fossil fuels, we have an impact on the carbon cycle (Figure below). This carbon is not recycled until it is used by humans. The burning of fossil fuels releases more carbon dioxide into the atmosphere than is used by photosynthesis. So, there is more carbon dioxide entering the atmosphere than is coming out of it. Carbon dioxide is known as a greenhouse gas, since it lets in light energy but does not let heat escape, much like the panes of a greenhouse. The increase of greenhouse gasses in the atmosphere is contributing to a global rise in Earth’s temperature, known as global warming or global climate change.

Summary
- During the carbon cycle, animals and plants add carbon dioxide to the atmosphere through cellular respiration, and plants remove carbon dioxide through photosynthesis.
- The burning of fossil fuels releases more carbon dioxide into the atmosphere, contributing to global warming.
Explore More
Use the resource below to answer the questions that follow.
- Organic Carbon and the World Around Us from USGS http://gallery.usgs.gov/videos/571#.UKWjAId9KSo (7:11)
- What are two types of carbon? What type is carbon dioxide (CO2)? What is an example of the other type?
- How can carbon aid the spread of toxic substances?
- Why are the reactivity and binding capabilities of carbon crucial to life?
Review
- What biological process releases carbon back into the atmosphere?
- What human activities have thrown the carbon cycle off balance?
- Why is carbon dioxide a greenhouse gas?
- What is the outcome of the increase of greenhouse gasses

