8.3: Extremophiles
- Page ID
- 5666
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Extremophiles
Extremophiles are organisms that live in habitats that seem extreme to humans. The ability of organisms on Earth to live in a wide range of environments is the secret to the survival of life on this planet and extremophiles help us to understand the limits to life. On the early Earth, the large swings in climate would have cycled between hot and freezing conditions. Before microbes invented photosynthesis, life adapted to take advantage of many different metabolic pathways. There is evidence in the geologic record that oxygen levels in the atmosphere fluctuated wildly before a precipitous rise 2.4 Gya. Through every mass extinction, there were some niches of life that survived. Even our flammable, oxygen-rich atmosphere constitutes an extreme environment.
Being "extreme" is in the eye of the beholder. Most of the organisms that humans identify as extremophiles are small prokaryotes in the Archaea or Bacteria domain. They are known to survive in a wide range of extraordinary environments.
Thermophiles (temperature-loving organisms) live in high temperature environments, some at temperatures of 235°F (113°C). Vast hydrothermal environments can be found on land surfaces and the deep seafloor. High temperatures are challenging for life because the energy causes proteins to denature, or unfold. Chemical reactions proceed more quickly and temperatures above 100∘C can denature nucleic acids, causing DNA to lose its helical shape, or break apart the structure of biomolecules. The fluidity and permeability of cell membranes is affected by heat. High temperatures decrease the solubility of carbon dioxide and oxygen in water, a problem for aquatic aerobic life. Thermophiles have metabolisms that take advantage of the higher chemical reactivity enabled by high temperatures. They make use of special temperature-resistant proteins and protective mechanisms for shielding DNA. Thermophile coping mechanisms include an altered ratio of saturated hydrocarbons in cell membranes.
The young Earth, with a tremendous amount of internal energy from accretion and differentiation may have harbored many hot springs and ocean floor hydrothermal vents. Young Earth would have been a paradise for thermophiles and the archea and bacteria that are most deeply rooted in the phylogenetic tree of life are thermophiles. The range of metabolisms for thermophiles may be the result of the diversity of chemistry at deep sea hydrothermal vents.
Psychrophiles are organisms that can withstand extreme cold. They are found in liquid brine inclusions in ice cores, or living under rocks in the extreme deserts of the world where temperatures can reach below -60°F (-50°C). This class of extremophiles includes bacteria and eukaryotes. Cells must be resilient to freezing, which can form ice shards that would pierce and destroy ordinary cells. One defense of a psychrophile is to lower the freezing point with solutes in cytoplasm that essentially act as an antifreeze. To increase fluidity of cell membranes, psychrophiles have evolved a different ratio of unsaturated to saturated fats. At cold temperatures, proteins are more rigid so enzymes are used to lower the activation energy for biochemical reactions.
Acidophiles and alkaliphiles thrive in environments with very low or high pH, respectively. Intense acids and bases are capable of breaking down organic chemicals and denaturing proteins. Acidophiles have been found in conditions as acidic as battery acid. They have been able to adapt mechanisms for keeping the acid out so that the cell cytoplasm can have a neutral pH.
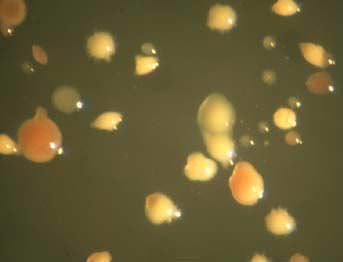
Life has even been found next to nuclear waste storage sites in the presence of enough radiation to grant super powers. Radiation can be very damaging to DNA and cause cancerous cells or tumors. These radioresistant organisms have evolved mechanisms to protect their DNA even in the presence of over 1000 times the radiation a typical organism can withstand.
There also still exist anaerobes that do not require oxygen and live deep underground. There are barophiles, or piezophiles that thrive in high pressure environments. Halophiles can only survive in an environment with high concentrations of salt, an environment that would dehydrate most other organisms. Zerophiles require very little water and are found in the soil of the world's largest deserts.
The discovery of extremophiles has greatly expanded our understanding of the range of environments that can host life. They are a great boon to astrobiology both in demonstrating the wide variety of life that is possible, and by expanding the definition of what is "habitable."
Tardigrades
Tardigrades, or water bears, are not technically extremophiles but are nevertheless extreme in their own right as well as strangely adorable. They are a type of micro-animal, only 0.5mm in length on average, with eight-legs, typically found in water, and among the most resilient creatures known. They are different from extremophiles, which are adapted to live in terrifyingly harsh conditions, because they do best in average conditions. However, they are capable of surviving the most extreme conditions on this world, including low pressure environments in space.

In 2007, samples of tardigrades were taken into low Earth orbit and exposed to the vacuum and radiation of space for 10 days. After reanimating the tardigrades back on Earth, most of the tardigrades began living as normal after 30 minutes (though most of the sample did suffer later health effects). Their incredible resilience allowed water bears to survive through five great extinction events on Earth. Their capability to survive in space gives new life to the theory of panspermia. Though not technically extremophiles, tardigrades certainly win a prize for resiliance.
In April 2019, an Israeli spacecraft called Beresheet almost made it to the moon. The privately funded mission was the first stage of a privately funded project to transfer DNA to the moon to build a repository for rebuilding life in the event of catastrophic mass extinction. Among the passengers on the spacecraft: dehydrated tardigrades. It is unlikely that these creatures could survive on the moon without liquid water.
Got Water?
Extremophiles occupy the entire range of environments it is possible to have on Earth. However, neither tardigrades nor any extremophile found yet can survive in the absence of water. Water is thought to be essential to life both because where there is life there is water and where there is water there is life. Water possess many unique chemical properties that make it conducive to both life and the organic reactions that govern it.
Water makes up the most of most living systems. The average organism is 70% or more water by weight. Recall that water, or H2O, consists of one oxygen molecule and two hydrogen molecules. As mentioned before, oxygen is particularly good at attracting electrons. Even within a molecule of water, the electrons preferentially spend time closer to the oxygen molecule than the hydrogen atoms. Because electrons carry a negative charge, the result is that the oxygen molecule is slightly negative while the hydrogen atoms are left with a slightly positive residual charge.
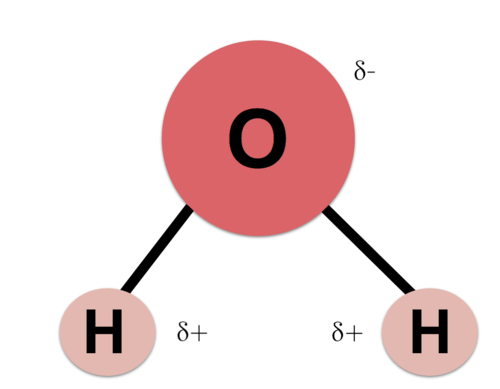
This charge differential across the water molecules makes water polar and consequently useful for organic chemistry in several ways. The different charges help group nonpolar molecules together and create effects like self-assembling phospholipid structures. Proteins are also folded with the help of water when amino acids with nonpolar side chains are naturally folded inside more water-friendly side chains.
Because of its polarity, water has a strong bond to neighboring water molecules. Water molecules can form hydrogen bonds with one another. These bonds help water to remain liquid over a greater range of temperature. Water provides a safe, stable environment to nurture the complex reactions required by life.
The difference in charge also allows water to more easily pull compounds apart, making it easier for chemicals (like the salt crystal in the figure below) to dissolve in water. This has earned water the moniker "the universal solvent." Once in the water, chemicals are free to move around and interact with other chemicals.
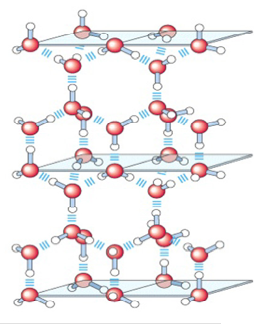
When water freezes into ice, the positively-charged hydrogen atoms align with the negatively-charged oxygen atoms, forming an organized lattice (figure below). In this highly-organized configuration, the molecules in ice are more widely spaced than molecules in water, resulting in a lower density that allows ice to float on top of water. This property carries many benefits for biology as ice can therefore serve as a protective barrier for the water underneath. It is capable of blocking out potentially damaging radiation and cooling water to stabilize certain compounds.
Icy Worlds
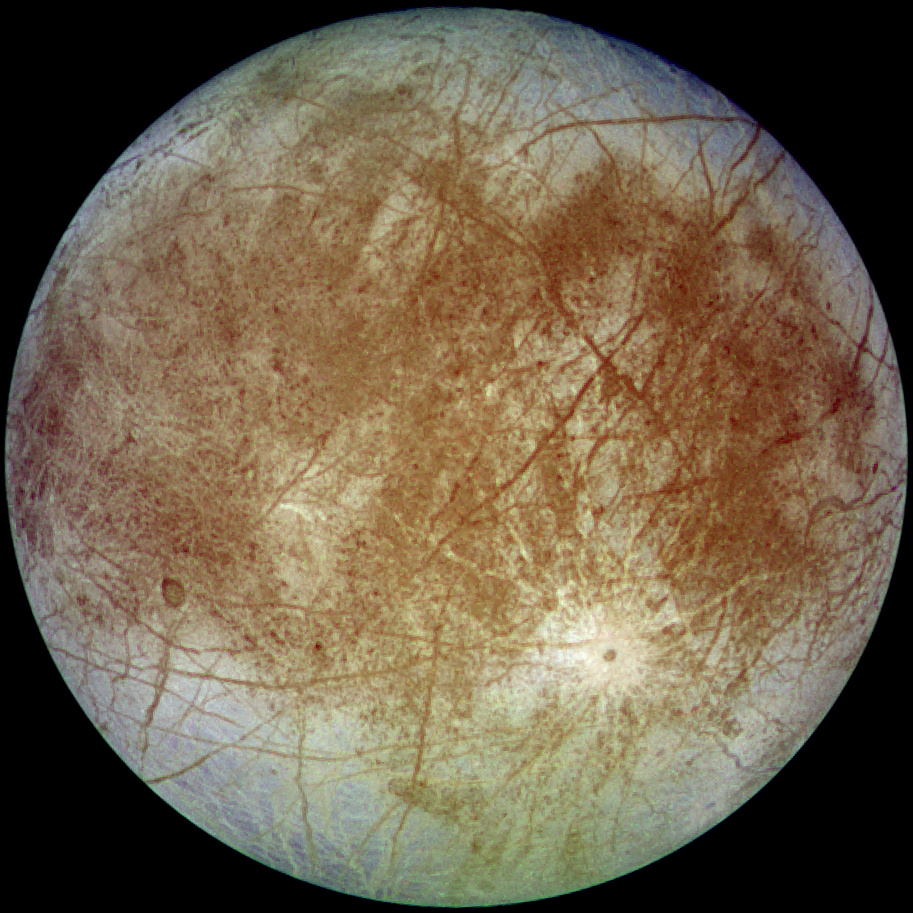
In recent years, the search for life in the solar system has expanded beyond Mars to also include some surprising places. Chief among them is a moon of Jupiter named Europa. Pictures of Europa reveal an exceedingly smooth surface with only cracks, no craters. For this reason, it is believed that Europa may be an ocean world covered by a layer of ice capable of recovering from impacts. Because of the close association of water to life, Europa is gaining popularity as the next target of a NASA mission to discover extraterrestrial life. As the wettest known world in the Solar System, Europa may be teaming with life just under the surface.
Saturn's moon Enceladus is also making a splash. Back in 2005, the Cassini spacecraft in orbit around Saturn discovered water geysers at the south pole of Enceladaus. It is thought that water from these geysers freezes into ice upon being released from Enceladus and adds to the ice in Saturn's rings, making them shinier. With a confirmed source of water under an icy surface, the story of Enceladus became even more exciting after the Cassini spacecraft maneuvered through one of the geysers on Enceladus. Cassini was able to detect high levels of hydrogen gas being expelled with the geyser. Though more information is needed, this preliminary finding suggests that geysers on Enceladus may resemble the deep-sea vents on Earth, thought to be the sites for the genesis of life.

The helpful properties of both water and ice are expanding the possibilities of what is deemed habitable. It might turn out that alien neighbors are closer than we thought.
Water is so essential for life as we know it that the search is on for sources and signs of water in space. Though water might not definitely point to life, it is hard to imagine life without water.

