2.33: Phospholipid Bilayer
- Page ID
- 1441
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)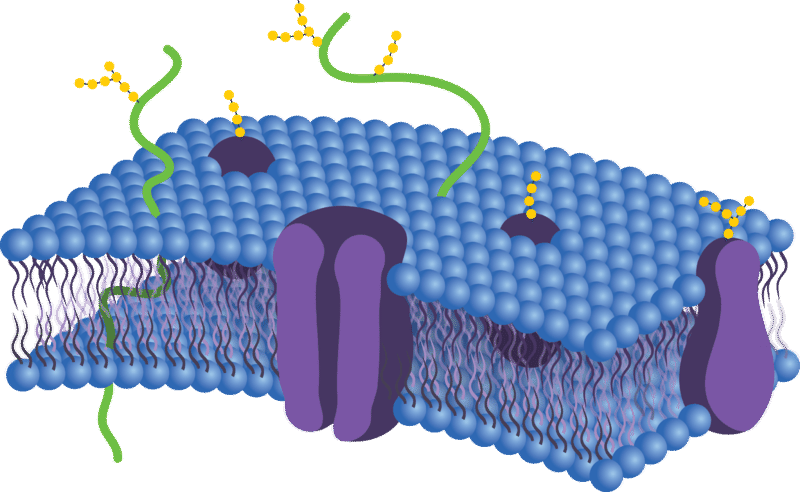
All cells have a plasma membrane. This membrane surrounds the cell. So what is its role?
Can molecules enter and leave the cell? Yes. Can anything or everything enter or leave? No. So, what determines what can go in or out? Is it the nucleus? The DNA? Or the plasma membrane?
The Plasma Membrane
The plasma membrane (also known as the cell membrane) forms a barrier between the cytoplasm inside the cell and the environment outside the cell. It protects and supports the cell and also controls everything that enters and leaves the cell. It allows only certain substances to pass through, while keeping others in or out. The ability to allow only certain molecules in or out of the cell is referred to as selective permeability or semipermeability. To understand how the plasma membrane controls what crosses into or out of the cell, you need to know its composition.
A Phospholipid Bilayer
The plasma membrane is composed mainly of phospholipids, which consist of fatty acids and alcohol. The phospholipids in the plasma membrane are arranged in two layers, called a phospholipid bilayer. As shown in the Figure below, each phospholipid molecule has a head and two tails. The head “loves” water (hydrophilic) and the tails “hate” water (hydrophobic). The water-hating tails are on the interior of the membrane, whereas the water-loving heads point outwards, toward either the cytoplasm or the fluid that surrounds the cell.
Molecules that are hydrophobic can easily pass through the plasma membrane, if they are small enough, because they are water-hating like the interior of the membrane. Molecules that are hydrophilic, on the other hand, cannot pass through the plasma membrane—at least not without help—because they are water-loving like the exterior of the membrane, and are therefore excluded from the interior of the membrane.
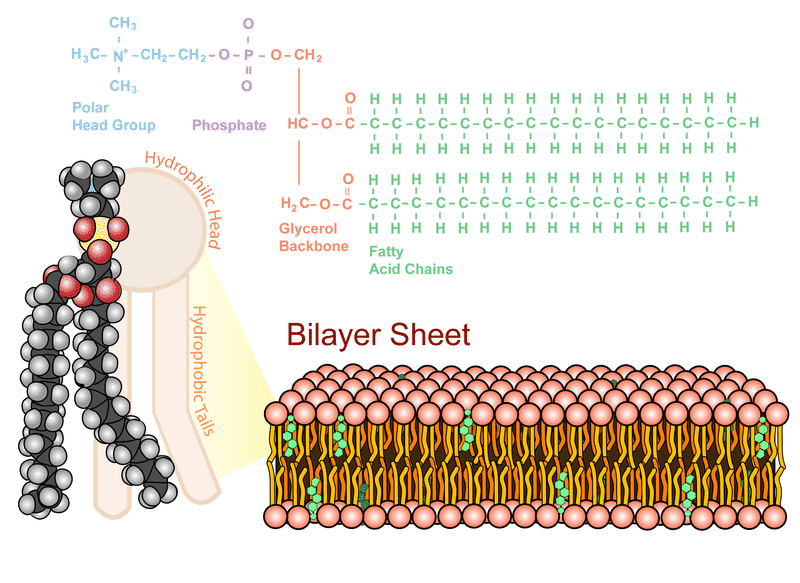 Phospholipid Bilayer. The phospholipid bilayer consists of two layers of phospholipids, with a hydrophobic, or water-hating, interior and a hydrophilic, or water-loving, exterior. The hydrophilic (polar) head group and hydrophobic tails (fatty acid chains) are depicted in the single phospholipid molecule. The polar head group and fatty acid chains are attached by a 3-carbon glycerol unit.
Phospholipid Bilayer. The phospholipid bilayer consists of two layers of phospholipids, with a hydrophobic, or water-hating, interior and a hydrophilic, or water-loving, exterior. The hydrophilic (polar) head group and hydrophobic tails (fatty acid chains) are depicted in the single phospholipid molecule. The polar head group and fatty acid chains are attached by a 3-carbon glycerol unit.Science Friday: Candy Corn in Space
Candy corn is a very tasty treat. In this video by Science Friday, astronaut Don Pettit uses Candy Corn to demonstrate the effects of hydrophobic and hydrophilic interactions.
Summary
- The plasma membrane forms a barrier between the cytoplasm and the environment outside the cell. The plasma membrane has selective permeability.
- The plasma membrane is primarily composed of phospholipids arranged in a bilayer, with the hydrophobic tails on the interior of the membrane, and the hydrophilic heads pointing outwards.
Review
- Describe the role of the plasma membrane.
- What is meant by semipermeability?
- Describe the composition of the plasma membrane.
- Explain why hydrophobic molecules can easily cross the plasma membrane, while hydrophilic molecules cannot.
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Flickr:stellarr Source: http://www.flickr.com/photos/21187899@N03/2058985902/ License: CC BY 2.0 |
 |
[Figure 2] | Credit: Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: CK-12 Foundation License: CC BY-NC 3.0 |

