1.1: Carbohydrates
- Page ID
- 8269
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Sugar. Does this look like biological energy?
As a child, you may have been told that sugar is bad for you. Well, that's not exactly true. Essentially, carbohydrates are made of sugar, from a single sugar molecule to thousands of sugar molecules attached together. Why? One reason is to store energy. But that does not mean you should eat it by the spoonful.
Carbohydrates
Carbohydrates are the most common type of organic compound. A carbohydrate is an organic compound such as sugar or starch, and is used to store energy. Like most organic compounds, carbohydrates are built of small, repeating units that form bonds with each other to make a larger molecule. In the case of carbohydrates, the small repeating units are called monosaccharides. Carbohydrates contain only carbon, hydrogen, and oxygen.
Monosaccharides and Disaccharides
A monosaccharide is a simple sugar such as fructose or glucose. Fructose is found in fruits, whereas glucose generally results from the digestion of other carbohydrates. Glucose (C6H12O6) is used for energy by the cells of most organisms, and is a product of photosynthesis.
The general formula for a monosaccharide is:
(CH2O)n,
where n can be any number greater than two. For example, in glucose n is 6, and the formula is:
C6H12O6.
Another monosaccharide, fructose, has the same chemical formula as glucose, but the atoms are arranged differently. Molecules with the same chemical formula but with atoms in a different arrangement are called isomers. Compare the glucose and fructose molecules in Figure below. Can you identify their differences? The only differences are the positions of some of the atoms. These differences affect the properties of the two monosaccharides.
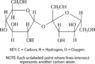
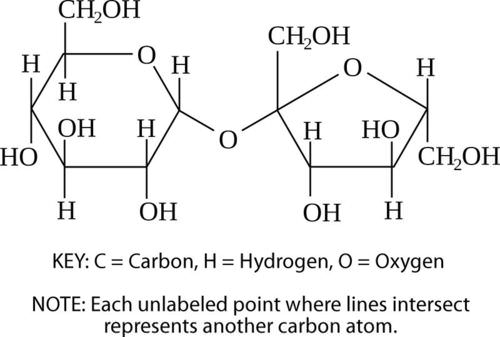 Sucrose Molecule. This sucrose molecule is a disaccharide. It is made up of two monosaccharides: glucose on the left and fructose on the right.
Sucrose Molecule. This sucrose molecule is a disaccharide. It is made up of two monosaccharides: glucose on the left and fructose on the right.If two monosaccharides bond together, they form a carbohydrate called a disaccharide. An example of a disaccharide is sucrose (table sugar), which consists of the monosaccharides glucose and fructose (Figure above). Monosaccharides and disaccharides are also called simple sugars. They provide the major source of energy to living cells
Polysaccharides
A polysaccharide is a complex carbohydrate that forms when simple sugars bind together in a chain. Polysaccharides may contain just a few simple sugars or thousands of them. Complex carbohydrates have two main functions: storing energy and forming structures of living things. Some examples of complex carbohydrates and their functions are shown in Table below. Which type of complex carbohydrate does your own body use to store energy?
| Name | Function | Example |
|---|---|---|
| Starch | Used by plants to store energy. |
 A potato stores starch in underground tubers. |
| Glycogen | Used by animals to store energy. |
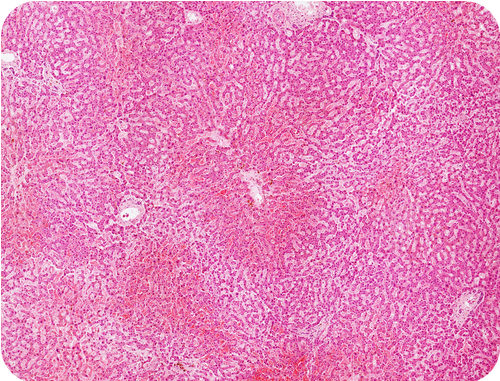 A human stores glycogen in liver cells. |
| Cellulose | Used by plants to form rigid walls around cells. |
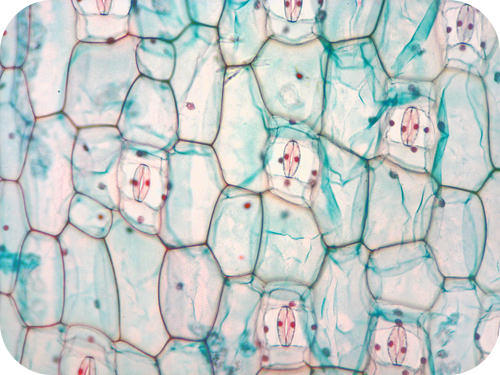 Plants use cellulose for their cell walls. |
| Chitin | Used by some animals to form an external skeleton. |
 A housefly uses chitin for its exoskeleton. |
Biofuels: From Sugar to Energy
For years there's been buzz, both positive and negative, about generating ethanol fuel from corn. Is this a good idea? Is it necessary? These questions need to be discussed. However, the Bay Area of California is rapidly becoming a world center for the next generation of green fuel alternatives. The Joint BioEnergy Institute is developing methods to isolate biofuels from the sugars in cellulose.
As you view Biofuels: Beyond Ethanol, focus on these concepts:
- the use of "cellulosic biomass,"
- what is meant by "directed evolution."
Summary
- Carbohydrates are organic compounds used to store energy.
- A monosaccharide is a simple sugar, such as fructose or glucose.
- Complex carbohydrates have two main functions: storing energy and forming structures of living things.
Review
- What is a carbohydrate?
- List three facts about glucose.
- Assume that you are trying to identify an unknown organic molecule. It contains only carbon, hydrogen, and oxygen and is found in the cell walls of a newly discovered plant species. What type of organic compound is it? Why?
- Compare and contrast the structures and functions of simple sugars and complex carbohydrates.
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: User:Booyabazooka/Wikimedia Commons Source: commons.wikimedia.org/wiki/File:Saccharose.svg License: CC BY-NC |
 |
[Figure 2] | Credit: User:Booyabazooka/Wikimedia Commons;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: commons.wikimedia.org/wiki/File:Saccharose.svg ; CK-12 Foundation License: Public Domain; CC BY-NC 3.0 |
 |
[Figure 3] | License: CC BY-NC |
 |
[Figure 4] | License: CC BY-NC |
 |
[Figure 5] | License: CC BY-NC |
 |
[Figure 6] | License: CC BY-NC |

