1.2: Proteins
- Page ID
- 8270
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
You may have been told proteins are good for you. Do these look good to you?
Proteins as food. To you, these may not look appetizing (or they might), but they do provide a nice supply of amino acids, the building blocks of proteins. Proteins have many important roles, from transporting, signaling, receiving, and catalyzing to storing, defending, and allowing for movement. Where do you get the amino acids needed so your cells can make their own proteins? If you cannot make it, you must eat it.
Proteins
A protein is an organic compound made up of small molecules called amino acids. There are 20 different amino acids commonly found in the proteins of living organisms. Small proteins may contain just a few hundred amino acids, whereas large proteins may contain thousands of amino acids. The largest known proteins are titins, found in muscle, which are composed from over 27,000 amino acids.
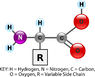
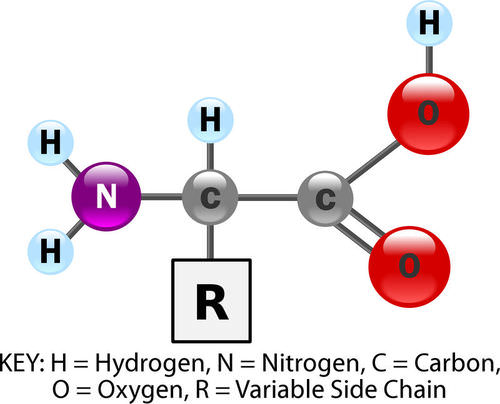 General Structure of Amino Acids. This model shows the general structure of all amino acids. Only the side chain, R, varies from one amino acid to another. For example, in the amino acid glycine, the side chain is simply hydrogen (H). In glutamic acid, in contrast, the side chain is CH2CH2COOH. Variable side chains give amino acids different chemical properties. The order of amino acids, together with the properties of the amino acids, determines the shape of the protein, and the shape of the protein determines the function of the protein. KEY: H = hydrogen, N = nitrogen, C = carbon, O = oxygen, R = variable side chain
General Structure of Amino Acids. This model shows the general structure of all amino acids. Only the side chain, R, varies from one amino acid to another. For example, in the amino acid glycine, the side chain is simply hydrogen (H). In glutamic acid, in contrast, the side chain is CH2CH2COOH. Variable side chains give amino acids different chemical properties. The order of amino acids, together with the properties of the amino acids, determines the shape of the protein, and the shape of the protein determines the function of the protein. KEY: H = hydrogen, N = nitrogen, C = carbon, O = oxygen, R = variable side chainProtein Structure
When amino acids bind together, they form a long chain called a polypeptide. A protein consists of one or more polypeptide chains. A protein may have up to four levels of structure. The lowest level, a protein’s primary structure, is its sequence of amino acids. Higher levels of protein structure are described in Figure below. The complex structures of different proteins give them unique properties, which they need to carry out their various jobs in living organisms.
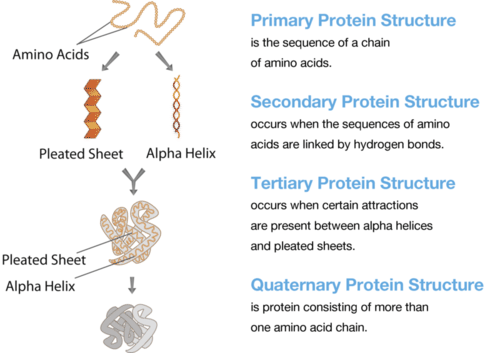 Protein Structure. The structure of a protein starts with its sequence of amino acids. What determines the secondary structure of a protein? What are two types of secondary protein structure?
Protein Structure. The structure of a protein starts with its sequence of amino acids. What determines the secondary structure of a protein? What are two types of secondary protein structure?Functions of Proteins
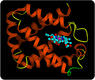
Proteins play many important roles in living things. Some proteins help cells keep their shape (structural proteins), some, such as connective and motor proteins, make up muscle tissues, and some transport items in and out of cells (transport proteins). Some proteins act as signals, and other proteins receive those signals. Enzymes are proteins that speed up chemical reactions in cells. Other proteins are antibodies, which bind to foreign substances such as bacteria and target them for destruction. Still other proteins carry messages or transport materials. For example, human red blood cells contain a protein called hemoglobin, which binds with oxygen. Hemoglobin allows the blood to carry oxygen from the lungs to cells throughout the body. A model of the hemoglobin molecule is shown in Figure below.
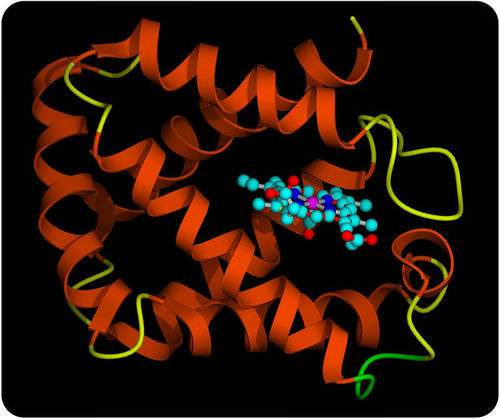 Hemoglobin Molecule. This model represents the protein hemoglobin. The purple part of the molecule contains iron. The iron binds with oxygen molecules.
Hemoglobin Molecule. This model represents the protein hemoglobin. The purple part of the molecule contains iron. The iron binds with oxygen molecules.Proteins and Diet
Proteins in the diet are necessary for life. Dietary proteins are broken down into their component amino acids when food is digested. Cells can then use the components to build new proteins. Humans are able to synthesize all but eight of the twenty common amino acids. These eight amino acids, called essential amino acids, must be consumed in foods. Like dietary carbohydrates and lipids, dietary proteins can also be broken down to provide cells with energy.
Science Friday: The Medical Wonders of Worm Spit
How useful is worm spit? It turns out that worm spit, also known as silk, is a very useful material in medicine. In this video by Science Friday, Dr. David Kaplan describes how silk is used in a variety of medical applications.
Summary
- Proteins are organic compounds made up of amino acids.
- A protein may have up to four levels of structure. The complex structures of different proteins give them unique properties.
- Enzymes are proteins that speed up biochemical reactions in cells. Antibodies are proteins that target pathogens for destruction.
Review
- Proteins are made out of ____________.
- What determines the primary structure of a protein?
- State two functions of proteins.
- What are enzymes?
- Describe the role of hemoglobin.
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: User:YassineMrabet/Wikimedia Commons;Image copyright ynse, 2014 Source: commons.wikimedia.org/wiki/File:AminoAcidball.svg ; http://www.shutterstock.com License: License from Shutterstock; CC BY-NC 3.0 |
 |
[Figure 2] | Credit: User:YassineMrabet/Wikimedia Commons;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: commons.wikimedia.org/wiki/File:AminoAcidball.svg ; CK-12 Foundation License: Public Domain; CC BY-NC 3.0 |
 |
[Figure 3] | Credit: Hana Zavadska, based on image from the National Human Genome Research Institute;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: CK-12 Foundation ; ck 12 foundation License: CC BY-NC 3.0 |
 |
[Figure 4] | Credit: Image copyright ynse, 2014;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: http://www.shutterstock.com ; CK-12 Foundation License: License from Shutterstock; CC BY-NC 3.0 |

