1.3: Lipids
- Page ID
- 8271
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Oil. Does it mix with water? No. Biologically, why is this important?
Oil is a lipid. The property of chemically not being able to mix with water gives lipids some very important biological functions. Lipids form the outer membrane of cells. Why?
Lipids
A lipid is an organic compound such as fat or oil. Organisms use lipids to store energy, but lipids have other important roles as well. Lipids consist of repeating units called fatty acids. Fatty acids are organic compounds that have the general formula CH3(CH2)nCOOH, where n usually ranges from 2 to 28 and is always an even number. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids.
Saturated Fatty Acids
In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. This causes the molecules to form straight chains, as shown in Figure below. The straight chains can be packed together very tightly, allowing them to store energy in a compact form. This explains why saturated fatty acids are solids at room temperature. Animals use saturated fatty acids to store energy.
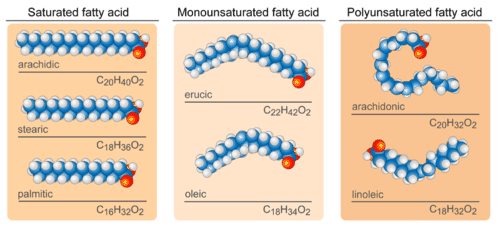 Fatty Acids. Saturated fatty acids have straight chains, like the three fatty acids shown in the upper left. Unsaturated fatty acids have bent chains, like all the other fatty acids in the figure.
Fatty Acids. Saturated fatty acids have straight chains, like the three fatty acids shown in the upper left. Unsaturated fatty acids have bent chains, like all the other fatty acids in the figure.Unsaturated Fatty Acids
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they are bonded to other groups of atoms. Wherever carbon binds with these other groups of atoms, it causes chains to bend (see Figure above). The bent chains cannot be packed together very tightly, so unsaturated fatty acids are liquids at room temperature. Plants use unsaturated fatty acids to store energy. Some examples are shown in Figure below.
 These plant products all contain unsaturated fatty acids.
These plant products all contain unsaturated fatty acids.Types of Lipids
Lipids may consist of fatty acids alone, or they may contain other molecules as well. For example, some lipids contain alcohol or phosphate groups. They include
- triglycerides: the main form of stored energy in animals.
- phospholipids: the major components of cell membranes.
- steroids: serve as chemical messengers and have other roles.
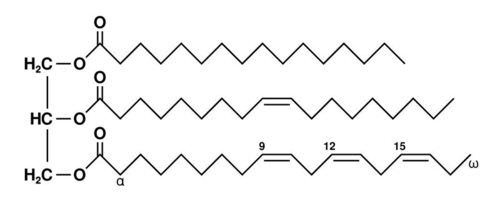 Triglyceride Molecule. The left part of this triglyceride molecule represents glycerol. Each of the three long chains on the right represents a different fatty acid. From top to bottom, the fatty acids are palmitic acid, oleic acid, and alpha-linolenic acid. The chemical formula for this triglyceride is C55H98O6. KEY:H=hydrogen, C=carbon, O=oxygen
Triglyceride Molecule. The left part of this triglyceride molecule represents glycerol. Each of the three long chains on the right represents a different fatty acid. From top to bottom, the fatty acids are palmitic acid, oleic acid, and alpha-linolenic acid. The chemical formula for this triglyceride is C55H98O6. KEY:H=hydrogen, C=carbon, O=oxygenLipids and Diet
Humans need lipids for many vital functions, such as storing energy and forming cell membranes. Lipids can also supply cells with energy. In fact, a gram of lipids supplies more than twice as much energy as a gram of carbohydrates or proteins. Lipids are necessary in the diet for most of these functions. Although the human body can manufacture most of the lipids it needs, there are others, called essential fatty acids, that must be consumed in food. Essential fatty acids include omega-3 and omega-6 fatty acids. Both of these fatty acids are needed for important biological processes, not just for energy.
Although some lipids in the diet are essential, excess dietary lipids can be harmful. Because lipids are very high in energy, eating too many may lead to unhealthy weight gain. A high-fat diet may also increase lipid levels in the blood. This, in turn, can increase the risk for health problems such as cardiovascular disease. The dietary lipids of most concern are saturated fatty acids, trans fats, and cholesterol. For example, cholesterol is the lipid mainly responsible for narrowing arteries and causing the disease atherosclerosis.
Summary
- Organisms use lipids to store energy. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids.
- Animals use saturated fatty acids to store energy. Plants use unsaturated fatty acids to store energy.
- Phospholipids are the major components of cell membranes.
- Excess dietary lipids can be harmful.
Review
- What is a lipid? Give three examples.
- Butter is a fat that is a solid at room temperature. What type of fatty acid does butter contain? How do you know?
- Explain why molecules of saturated and unsaturated fatty acids have different shapes.
- Which lipid is the main component of cell membranes?
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Wolfgang Schaefer;Seeds: Lebensmittelfotos; Olives: Steve Jurvetson; Nuts: Petr Kratochvil Source: commons.wikimedia.org/wiki/Image:Fat_triglyceride_shorthand_formula.PNG ; Seeds: pixabay.com/en/barley-grain-cereals-whole-wheat-74247/ ; Olives: http://www.flickr.com/photos/jurvetson/454873761/ ; Nuts: http://www.publicdomainpictures.net/view-image.php?image=424& ; picture=nuts License: Public Domain; Seeds: Public Domain; Olives: CC BY 2.0; Nuts: Public Domain |
 |
[Figure 2] | Credit: Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation;CK-12 Foundation - Joy Sheng;By Smokefoot - Own work, Public Domain, commons.wikimedia.org/w/inde...90004;Wolfgang Schaefer Source: CK-12 Foundation ; By Smokefoot - Own work ; Public Domain ; commons.wikimedia.org/w/index.php?curid=11990004 ; commons.wikimedia.org/wiki/Image:Fat_triglyceride_shorthand_formula.PNG License: CC BY-NC 3.0; Public Domain |
 |
[Figure 3] | Credit: Seeds: Lebensmittelfotos; Olives: Steve Jurvetson; Nuts: Petr Kratochvil;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation;Wolfgang Schaefer Source: Seeds: pixabay.com/en/barley-grain-cereals-whole-wheat-74247/ ; Olives: http://www.flickr.com/photos/jurvetson/454873761/ ; Nuts: http://www.publicdomainpictures.net/view-image.php?image=424& ; picture=nuts ; CK-12 Foundation ; eeds: pixabay.com/en/barley-grain-cereals-whole-wheat-74247/ ; Olives: http://www.flickr.com/photos/jurvetson/454873761/ ; Nuts: http://www.publicdomainpictures.net/view-image.php?image=424& ; picture=nuts ; commons.wikimedia.org/wiki/Image:Fat_triglyceride_shorthand_formula.PNG License: Seeds: Public Domain; Olives: CC BY 2.0; Nuts: Public Domain; CC BY-NC 3.0; Public Domain |
 |
[Figure 4] | Credit: Wolfgang Schaefer;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation;CK-12 Foundation - Joy Sheng Source: commons.wikimedia.org/wiki/Image:Fat_triglyceride_shorthand_formula.PNG ; CK-12 Foundation License: Public Domain; CC BY-NC 3.0 |

