1.9: Enzyme Function
- Page ID
- 8342
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Do cells have one enzyme with lots of functions, or many enzymes, each with just one function?
Enzymes. Vital proteins necessary for life. So how do enzymes work? How do they catalyze just one specific biochemical reaction? In a puzzle, only two pieces will fit together properly. Understanding that is one of the main steps in understanding how enzymes work.
Enzyme Function
How do enzymes speed up biochemical reactions so dramatically? Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. Activation energy is the energy needed to start a chemical reaction. This is illustrated in Figure below. The biochemical reaction shown in the figure requires about three times as much activation energy without the enzyme as it does with the enzyme.
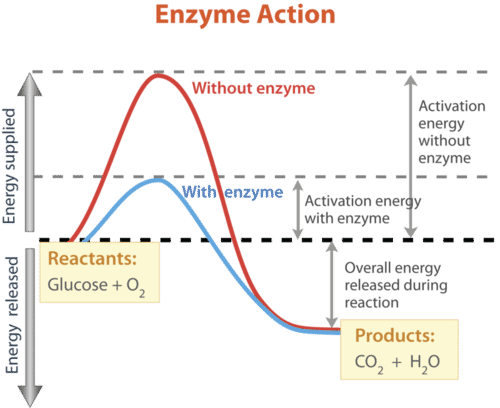
The reaction represented by this graph is a combustion reaction involving the reactants glucose (C6H12O6) and oxygen (O2). The products of the reaction are carbon dioxide (CO2) and water (H2O). Energy is also released during the reaction. The enzyme speeds up the reaction by lowering the activation energy needed for the reaction to start. Compare the activation energy with and without the enzyme.
Enzymes generally lower activation energy by reducing the energy needed for reactants to come together and react. For example:
- Enzymes bring reactants together so they don’t have to expend energy moving about until they collide at random. Enzymes bind both reactant molecules (called the substrate), tightly and specifically, at a site on the enzyme molecule called the active site (Figure below).
- By binding reactants at the active site, enzymes also position reactants correctly, so they do not have to overcome intermolecular forces that would otherwise push them apart. This allows the molecules to interact with less energy.
- Enzymes may also allow reactions to occur by different pathways that have lower activation energy.
The active site is specific for the reactants of the biochemical reaction the enzyme catalyzes. Similar to puzzle pieces fitting together, the active site can only bind certain substrates.
 This enzyme molecule binds reactant molecules—called substrate—at its active site, forming an enzyme-substrate complex. This brings the reactants together and positions them correctly so the reaction can occur. After the reaction, the products are released from the enzyme’s active site. This frees up the enzyme so it can catalyze additional reactions.
This enzyme molecule binds reactant molecules—called substrate—at its active site, forming an enzyme-substrate complex. This brings the reactants together and positions them correctly so the reaction can occur. After the reaction, the products are released from the enzyme’s active site. This frees up the enzyme so it can catalyze additional reactions.The activities of enzymes also depend on the temperature, ionic conditions, and the pH of the surroundings. Some enzymes work best at acidic pHs, while others work best in neutral environments.
- Digestive enzymes secreted in the acidic environment (low pH) of the stomach help break down proteins into smaller molecules. The main digestive enzyme in the stomach is pepsin, which works best at a pH of about 1.5. These enzymes would not work optimally at other pHs. Trypsin is another enzyme in the digestive system, which breaks protein chains in food into smaller parts. Trypsin works in the small intestine, which is not an acidic environment. Trypsin's optimum pH is about 8.
- Biochemical reactions are optimal at physiological temperatures. For example, most biochemical reactions work best at the normal body temperature of 98.6˚F. Many enzymes lose function at lower and higher temperatures. At higher temperatures, an enzyme’s shape deteriorates. Only when the temperature comes back to normal does the enzyme regain its shape and normal activity.
Summary
- Enzymes work by lowering the activation energy needed to start biochemical reactions.
- The activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings.
Review
- How do enzymes speed up biochemical reactions?
- Where is the active site located? Explain the role of the active site?
- Complete this sentence: The activities of enzymes depends on the __________, __________ conditions, and the __________ of the surroundings.
- Distinguish between the conditions needed for the proper functioning of pepsin and trypsin.
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Jerry Crimson Mann (Wikimedia: Mcy jerry);Hana Zavadska;Laura Guerin Source: commons.wikimedia.org/wiki/File:Allosteric_competitive_inhibition_3.svg ; CK-12 Foundation License: CC BY-NC 3.0 |
 |
[Figure 2] | Credit: Hana Zavadska;Jerry Crimson Mann (Wikimedia: Mcy jerry) Source: CK-12 Foundation ; commons.wikimedia.org/wiki/File:Allosteric_competitive_inhibition_3.svg License: CC BY-NC 3.0 |
 |
[Figure 3] | Credit: Laura Guerin;Jerry Crimson Mann (Wikimedia: Mcy jerry) Source: CK-12 Foundation ; commons.wikimedia.org/wiki/File:Allosteric_competitive_inhibition_3.svg License: CC BY-NC 3.0 |

