1.10: Water and Life
- Page ID
- 8343
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Dihydrogen oxide, or dihydrogen monoxide. Does this chemical sound dangerous?
Another name for this compound is…water. Water can create some absolutely beautiful sights. Iguassu Falls is the largest series of waterfalls on the planet, located in Brazil, Argentina, and Paraguay. And water is necessary for life. The importance of water to life cannot be emphasized enough. All life needs water. Life started in water. Essentially, without this simple three atom molecule, life would not exist.
Water
Water, like carbon, has a special role in living things. It is needed by all known forms of life. Water is a simple molecule, containing just three atoms. Nonetheless, water’s structure gives it unique properties that help explain why it is vital to all living organisms.
Water, Water Everywhere
Water is a common chemical substance on planet Earth. In fact, Earth is sometimes called the "water planet" because almost 75% of its surface is covered with water. If you look at Figure below, you will see where Earth’s water is found. The term water generally refers to its liquid state, and water is a liquid over a wide range of temperatures on Earth. However, water also occurs on Earth as a solid (ice) and as a gas (water vapor).
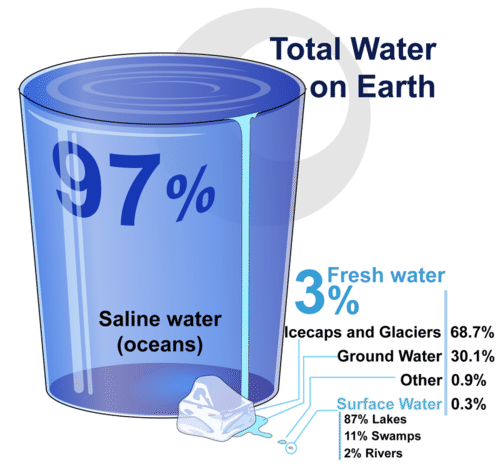 Most of the water on Earth consists of saltwater in the oceans. What percent of Earth’s water is fresh water? Where is most of the fresh water found?
Most of the water on Earth consists of saltwater in the oceans. What percent of Earth’s water is fresh water? Where is most of the fresh water found?Structure and Properties of Water
No doubt, you are already aware of some of the properties of water. For example, you probably know that water is tasteless and odorless. You also probably know that water is transparent, which means that light can pass through it. This is important for organisms that live in the water, because some of them need sunlight to make food.
Chemical Structure of Water
To understand some of water’s properties, you need to know more about its chemical structure. As you have seen, each molecule of water consists of one atom of oxygen and two atoms of hydrogen. The oxygen atom in a water molecule attracts negatively-charged electrons more strongly than the hydrogen atoms do. As a result, the oxygen atom has a slightly negative charge, and the hydrogen atoms have a slightly positive charge. A difference in electrical charge between different parts of the same molecule is called polarity, making water a polar molecule. The diagram in Figure below shows water’s polarity.
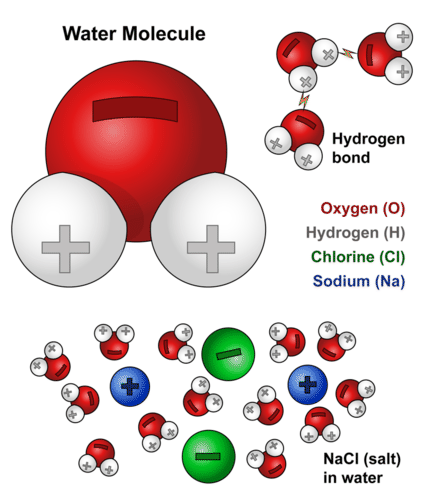 Water Molecule. This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule.
Water Molecule. This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule.Opposites attract when it comes to charged molecules. In the case of water, the positive (hydrogen) end of one water molecule is attracted to the negative (oxygen) end of a nearby water molecule. Because of this attraction, weak bonds form between adjacent water molecules, as shown in Figure below. The type of bond that forms between molecules is called a hydrogen bond. Bonds between molecules are not as strong as bonds within molecules. There are just many more hydrogen bonds in water (between water molecules) than there are covalent bonds within a molecule. The hydrogen bonds may not be strong, but in water they are strong enough to hold together nearby molecules.
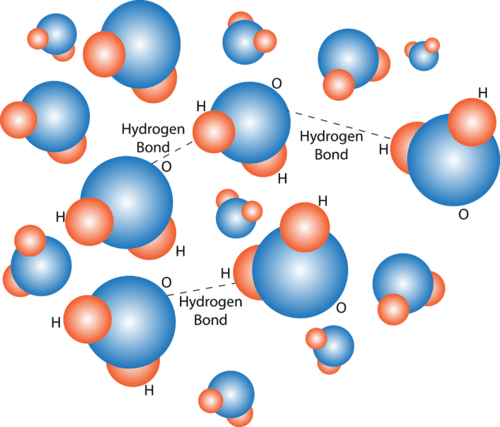 Hydrogen Bonding in Water Molecules. Hydrogen bonds form between nearby water molecules. How do you think this might affect water’s properties?
Hydrogen Bonding in Water Molecules. Hydrogen bonds form between nearby water molecules. How do you think this might affect water’s properties?Properties of Water
Hydrogen bonds between water molecules explain some of water’s properties. For example, hydrogen bonds explain why water molecules tend to stick together. Have you ever watched water drip from a leaky faucet or from a melting icicle? If you have, then you know that water always falls in drops rather than as separate molecules. The dew drops in Figure below are another example of water molecules sticking together.
 Droplets of Dew. Drops of dew cling to a spider web in this picture. Can you think of other examples of water forming drops? (Hint: What happens when rain falls on a newly waxed car?)
Droplets of Dew. Drops of dew cling to a spider web in this picture. Can you think of other examples of water forming drops? (Hint: What happens when rain falls on a newly waxed car?)Hydrogen bonds cause water to have a relatively high boiling point of 100°C (212°F). Because of its high boiling point, most water on Earth is in a liquid state rather than in a gaseous state. Water in its liquid state is needed by all living things. Hydrogen bonds also cause water to expand when it freezes. This, in turn, causes ice to have a lower density (mass/volume) than liquid water. The lower density of ice means that it floats on water. For example, in cold climates, ice floats on top of the water in lakes. This allows lake animals such as fish to survive the winter by staying in the water under the ice.
Water and Life
The human body is about 70% water (not counting the water in body fat, which varies from person to person). The body needs all this water to function normally. Just why is so much water required by human beings and other organisms? Water can dissolve many substances that organisms need, and it is necessary for many biochemical reactions. The examples below are among the most important biochemical processes that occur in living things, but they are just two of many ways that water is involved in biochemical reactions.
- Photosynthesis—In this process, cells use the energy in sunlight to change carbon dioxide and water to glucose and oxygen. Water is a reactant in this process. The reactions of photosynthesis can be represented by the chemical equation
6CO2 + 6H2O + Energy → C6H12O6 + 6O2.
- Cellular respiration—In this process, cells break down glucose in the presence of oxygen and release carbon dioxide, water (a product), and energy. The reactions of cellular respiration can be represented by the chemical equation
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy.
Water is involved in many other biochemical reactions. As a result, just about all life processes depend on water. Clearly, life as we know it could not exist without water.
Summary
- Water is needed by all known forms of life.
- Due to the difference in the distribution of charge, water is a polar molecule.
- Hydrogen bonds hold adjacent water molecules together.
- Water is involved in many biochemical reactions. As a result, just about all life processes depend on water.
Review
- Where is most of Earth’s water found?
- What percent of Earth’s water is fresh water?
- What is polarity? Describe the polarity of water.
- How could you demonstrate to a child that solid water is less dense than liquid water?
- Explain how water’s polarity is related to its boiling point.
- Explain why metabolism in organisms depends on water.
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Flickr:devra Source: http://www.flickr.com/photos/minicooper93402/6181554829/ License: CC BY-NC 3.0 |
 |
[Figure 2] | Credit: Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: CK-12 Foundation License: CC BY-NC 3.0 |
 |
[Figure 3] | Credit: Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: CK-12 Foundation License: CC BY-NC 3.0 |
 |
[Figure 4] | Credit: Jodi So Source: CK-12 Foundation License: CC BY-NC 3.0 |
 |
[Figure 5] | Credit: Flickr:devra;Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation Source: http://www.flickr.com/photos/minicooper93402/6181554829/ ; CK-12 Foundation License: CC BY 2.0; CC BY-NC 3.0 |

