6.27: Carbon Cycle
- Page ID
- 12125
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
How could releasing this much pollution into the atmosphere not be a poor idea?
Burning fossil fuels, such as oil, releases carbon into the atmosphere. This carbon must be cycled - removed from the atmosphere - back into living organisms, or it stays in the atmosphere. Increased carbon in the atmosphere contributes to the greenhouse effect on Earth.
The Carbon Cycle
Flowing water can slowly dissolve carbon in sedimentary rock. Most of this carbon ends up in the ocean. The deep ocean can store carbon for thousands of years or more. Sedimentary rock and the ocean are major reservoirs of stored carbon. Carbon is also stored for varying lengths of time in the atmosphere, in living organisms, and as fossil fuel deposits. These are all parts of the carbon cycle, which is shown in Figure below.
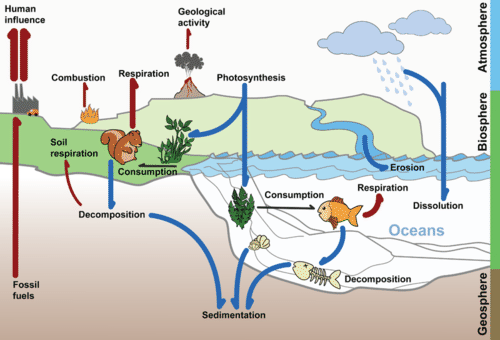 The Carbon Cycle. Carbon moves from one reservoir to another in the carbon cycle. What role do organisms play in this cycle?
The Carbon Cycle. Carbon moves from one reservoir to another in the carbon cycle. What role do organisms play in this cycle?Why is recycling carbon important? Recall that carbon is the cornerstone of organic compounds, the compounds necessary for life. But do organisms make their own carbon? Do they have the genes that encode proteins necessary to make carbon? No. In fact, there are no such genes. Carbon must be recycled from other living organisms, from carbon in the atmosphere, and from carbon in other parts of the biosphere.
Carbon in the Atmosphere
Though carbon can be found in ocean water, rocks and sediment and other parts of the biosphere, the atmosphere may be the most recognizable reservoir of carbon. Carbon occurs in various forms in different parts of the carbon cycle. Some of the different forms in which carbon appears are described in Table below. KEY: C = Carbon, O = Oxygen, H = Hydrogen
| Form of Carbon | Chemical Formula | State | Main Reservoir |
|---|---|---|---|
| Carbon Dioxide | CO2 | Gas | Atmosphere |
| Carbonic Acid | H2CO3 | Liquid | Ocean |
| Bicarbonate Ion | HCO3− | Liquid(dissolved ion) | Ocean |
| Organic Compounds | Examples: C6H12O6 (Glucose), CH4 (Methane) | Solid Gas | Biosphere Organic Sediments (Fossil Fuels) |
| Other Carbon Compounds | Examples: CaCO3 (Calcium Carbonate), CaMg(CO3)2 (Calcium Magnesium Carbonate) | Solid Solid | Sedimentary Rock, Shells, Sedimentary Rock |
Carbon in Carbon Dioxide
Carbon cycles quickly between organisms and the atmosphere. In the atmosphere, carbon exists primarily as carbon dioxide (CO2). Carbon dioxide cycles through the atmosphere by several different processes, including those listed below.
- Living organisms release carbon dioxide as a byproduct of cellular respiration.
- Photosynthesis removes carbon dioxide from the atmosphere and uses it to make organic compounds.
- Carbon dioxide is given off when dead organisms and other organic materials decompose.
- Burning organic material, such as fossil fuels, releases carbon dioxide.
- Carbon cycles far more slowly through geological processes such as sedimentation. Carbon may be stored in sedimentary rock for millions of years.
- When volcanoes erupt, they give off carbon dioxide that is stored in the mantle.
- Carbon dioxide is released when limestone is heated during the production of cement.
- Ocean water releases dissolved carbon dioxide into the atmosphere when water temperature rises.
- Carbon dioxide is also removed when ocean water cools and dissolves more carbon dioxide from the air.
Because of human activities, there is more carbon dioxide in the atmosphere today than in the past hundreds of thousands of years. Burning fossil fuels has released great quantities of carbon dioxide into the atmosphere. Cutting forests and clearing land has also increased carbon dioxide into the atmosphere because these activities reduce the number of autotrophic organisms that use up carbon dioxide in photosynthesis. In addition, clearing often involves burning, which releases carbon dioxide that was previously stored in autotrophs.
Summary
- Carbon must be recycled through living organisms or it stays in the atmosphere.
- Carbon cycles quickly between organisms and the atmosphere.
- Due to human activities, there is more carbon dioxide in the atmosphere today than in the past hundreds of thousands of years.
Review
- What is the role of the carbon cycle?
- Why is cycling carbon important?
- Describe a major method that carbon is cycled.
- How have human activities increased atmospheric carbon dioxide levels?
| Image | Reference | Attributions |
 |
[Figure 1] | Credit: Mariana Ruiz Villarreal (LadyofHats) for CK-12 Foundation;Stomata: Dartmouth Electron Microscope Facility; Leaf: Jon Sullivan Source: CK-12 Foundation ; Stomata: commons.wikimedia.org/wiki/File:Tomato_leaf_stomate_1-color.jpg ; Leaf: commons.wikimedia.org/wiki/File:Leaf_1_web.jpg License: Public Domain; CC BY-NC 3.0 |
 |
[Figure 2] | Credit: Mariana Ruiz Villarreal (LadyofHats) for CK-12 Foundation;Amy Goldstein;Jami Dwyer Source: CK-12 Foundation ; http://www.flickr.com/photos/amylovesyah/3945525048/ ; commons.wikimedia.org/wiki/File:Lacanja_burn.JPG License: CC BY-NC 3.0; CC BY 2.0; Public Domain |

