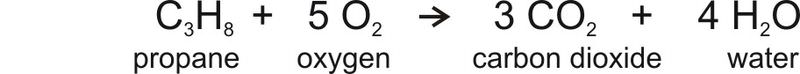12.16: Carbon Cycle and Climate
- Page ID
- 5543
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What is a diamond?
Carbon takes all sorts of forms as an element and as a compound. A diamond is just carbon, pure carbon. A diamond is good for cutting things, but it's not good for breathing or building proteins out of, yet other forms of carbon are. Carbon is essential for life on Earth and, as carbon dioxide, it is an important atmospheric gas.
The Carbon Cycle
Carbon is a very important element to living things. As the second most common element in the human body, we know that human life without carbon would not be possible. Protein, carbohydrates, and fats are all part of the body and all contain carbon. When your body breaks down food to produce energy, you break down protein, carbohydrates, and fat, and you breathe out carbon dioxide.
Carbon occurs in many forms on Earth. The element moves through organisms and then returns to the environment. When all this happens in balance, the ecosystem remains in balance too.
Short-Term Cycling of Carbon
The short term cycling of carbon begins with carbon dioxide (CO2) in the atmosphere.
Photosynthesis
Through photosynthesis, the inorganic carbon in carbon dioxide plus water and energy from sunlight is transformed into organic carbon (food) with oxygen given off as a waste product. The chemical equation for photosynthesis is:
Respiration
Plants and animals engage in the reverse of photosynthesis, which is respiration. In respiration, animals use oxygen to convert the organic carbon in sugar into food energy they can use. Plants also go through respiration and consume some of the sugars they produce.
The chemical reaction for respiration is:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + useable energy
Photosynthesis and respiration are a gas exchange process. In photosynthesis, CO2 is converted to O2; in respiration, O2 is converted to CO2.
Remember that plants do not create energy. They change the energy from sunlight into chemical energy that plants and animals can use as food (Figure below).
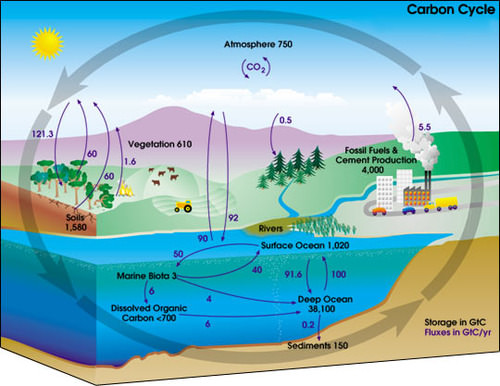
The carbon cycle shows where a carbon atom might be found. The black numbers indicate how much carbon is stored in various reservoirs, in billions of tons ("GtC" stands for gigatons of carbon). The purple numbers indicate how much carbon moves between reservoirs each year. The sediments, as defined in this diagram, do not include the ~70 million GtC of carbonate rock and kerogen.
Long-Term Carbon Cycling
Carbon Sinks and Carbon Sources
Places in the ecosystem that store carbon are reservoirs. Places that supply and remove carbon are carbon sources and carbon sinks, respectively. If more carbon is provided than stored, the place is a carbon source. If more carbon dioxide is absorbed than is emitted, the reservoir is a carbon sink. What are some examples of carbon sources and sinks?
- Carbon sinks are reservoirs where carbon is stored. Healthy living forests and the oceans act as carbon sinks.
- Carbon sources are reservoirs from which carbon can enter the environment. The mantle is a source of carbon from volcanic gases.
A reservoir can change from a sink to a source and vice versa. A forest is a sink, but when the forest burns it becomes a source.
The amount of time that carbon stays, on average, in a reservoir is the residence time of carbon in that reservoir.
Atmospheric Carbon Dioxide
Remember that the amount of CO2 in the atmosphere is very low. This means that a small increase or decrease in the atmospheric CO2 can have a large effect.
By measuring the composition of air bubbles trapped in glacial ice, scientists can learn the amount of atmospheric CO2 at times in the past. Of particular interest is the time just before the Industrial Revolution, when society began to use fossil fuels. That value is thought to be the natural content of CO2 for this time period; that number was 280 parts per million (ppm).
By 1958, when scientists began to directly measure CO2 content from the atmosphere at Mauna Loa volcano in the Pacific Ocean, the amount was 316 ppm (Figure below). In 2018, the atmospheric CO2 content crossed over to 410 ppm.

The amount of CO2 in the atmosphere has been measured at Mauna Loa Observatory since 1958. The blue line shows yearly averaged CO2. The red line shows seasonal variations in CO2.
This is an increase in atmospheric CO2 of 40% since the before the Industrial Revolution. About 65% of that increase has occurred since the first CO2 measurements were made on Mauna Loa Volcano, Hawaii, in 1958.
Human Actions Impact the Carbon Cycle
Humans have changed the natural balance of the carbon cycle because we use coal, oil, and natural gas to supply our energy demands. Fossil fuels are a sink for CO2 when they form, but they are a source for CO2 when they are burned.
The equation for combustion of propane, a simple hydrocarbon, looks like this:
The equation shows that when propane burns, it uses oxygen and produces carbon dioxide and water. So when a car burns a tank of gas, the amount of CO2 in the atmosphere increases just a little. Added over millions of tanks of gas and coal burned for electricity in power plants and all of the other sources of CO2, the result is the increase in atmospheric CO2 seen in the Figure above.
The second largest source of atmospheric CO2 is deforestation (Figure below). Trees naturally absorb CO2 while they are alive. Trees that are cut down lose their ability to absorb CO2. If the tree is burned or decomposes, it becomes a source of CO2. A forest can go from being a carbon sink to being a carbon source.
This forest in Mexico has been cut down and burned to clear forested land for agriculture.
Why the Carbon Cycle is Important
Why is such a small amount of carbon dioxide in the atmosphere even important? Carbon dioxide is a greenhouse gas. Greenhouse gases trap thermal energy that would otherwise radiate out into space, which warms Earth. These gases were discussed in the chapter Atmospheric Processes.
Summary
- Carbon is essential for life as part of proteins, carbohydrates, and fats.
- The amount of carbon dioxide in the atmosphere is extremely low, but it is extremely important since carbon dioxide is a greenhouse gas, which helps to keep Earth's climate moderate.
- The amount of carbon dioxide in the atmosphere is rising, a fact that has been documented on Mauna Loa volcano since 1958.
Review
- What does it mean to say that photosynthesis and respiration are gas exchange processes?
- How do scientists learn about carbon levels in the past?
- How do human activities affect the carbon cycle?
Explore More
Use this resource to answer the questions that follow.
- What do greenhouse gases do?
- Where did most of the carbon dioxide that was present in the early atmosphere go?
- What did the early plants add to the atmosphere and why was that important? What else did they create?
- What do organisms do with the organic carbon?
- What are the two major things that carbon does?
- What is the 30 second version of the carbon cycle?
- What does carbon fixation do with carbon dioxide?
- How do organisms use the carbohydrates produced by carbon fixing reactions?
- What is cellular respiration the reverse of?
- After the organisms metabolize carbohydrates, how is the carbon released back into the environment?
- What happens when carbon dioxide mixes with water and what does it cause?
- What happens to the carbonate ions in the marine environment?
- What happens when shell building organisms die? What happens if those organisms are buried deeply?
- How much carbon is wrapped up in fossil fuels compared to the total amount of carbon?
- Where does the carbon dioxide go that is released from fossil fuels? Where does the excess carbon dioxide go?
Resources
Tags recommended by the template: article:topic